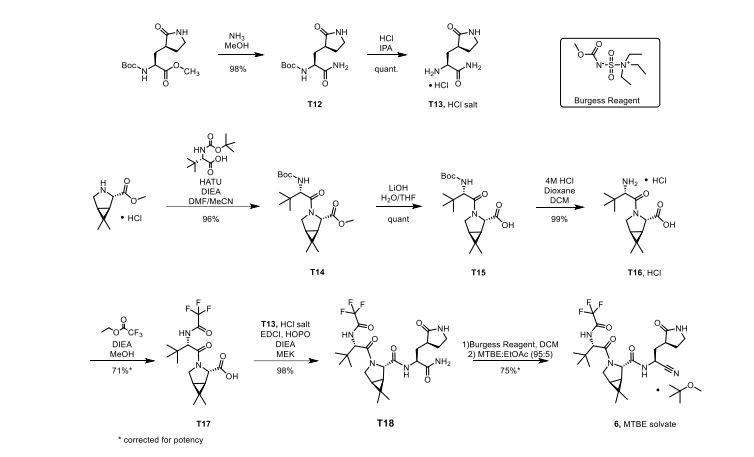
PF-07321332, nirmatrelvir
UNII-7R9A5P7H32, 7R9A5P7H32, PF07321332
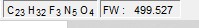
CAS 2628280-40-8, C23H32F3N5O4, 499.5
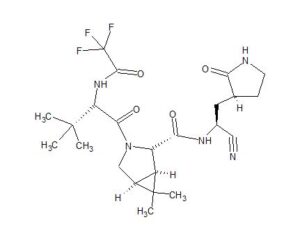
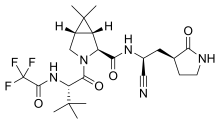
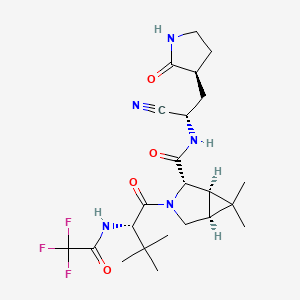
(1R,2S,5S)-N-[(1S)-1-cyano-2-[(3S)-2-oxopyrrolidin-3-yl]ethyl]-3-[(2S)-3,3-dimethyl-2-[(2,2,2-trifluoroacetyl)amino]butanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
https://clinicaltrials.gov/ct2/show/NCT04756531

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
SYN
https://pubmed.ncbi.nlm.nih.gov/34726479/
https://www.science.org/doi/10.1126/science.abl4784
Science. 2021 Dec 24;374(6575):1586-1593.
doi: 10.1126/science.abl4784. Epub 2021 Nov 2.
An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19
The worldwide outbreak of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic. Alongside vaccines, antiviral therapeutics are an important part of the healthcare response to countering the ongoing threat presented by COVID-19. Here, we report the discovery and characterization of PF-07321332, an orally bioavailable SARS-CoV-2 main protease inhibitor with in vitro pan-human coronavirus antiviral activity and excellent off-target selectivity and in vivo safety profiles. PF-07321332 has demonstrated oral activity in a mouse-adapted SARS-CoV-2 model and has achieved oral plasma concentrations exceeding the in vitro antiviral cell potency in a phase 1 clinical trial in healthy human participants.
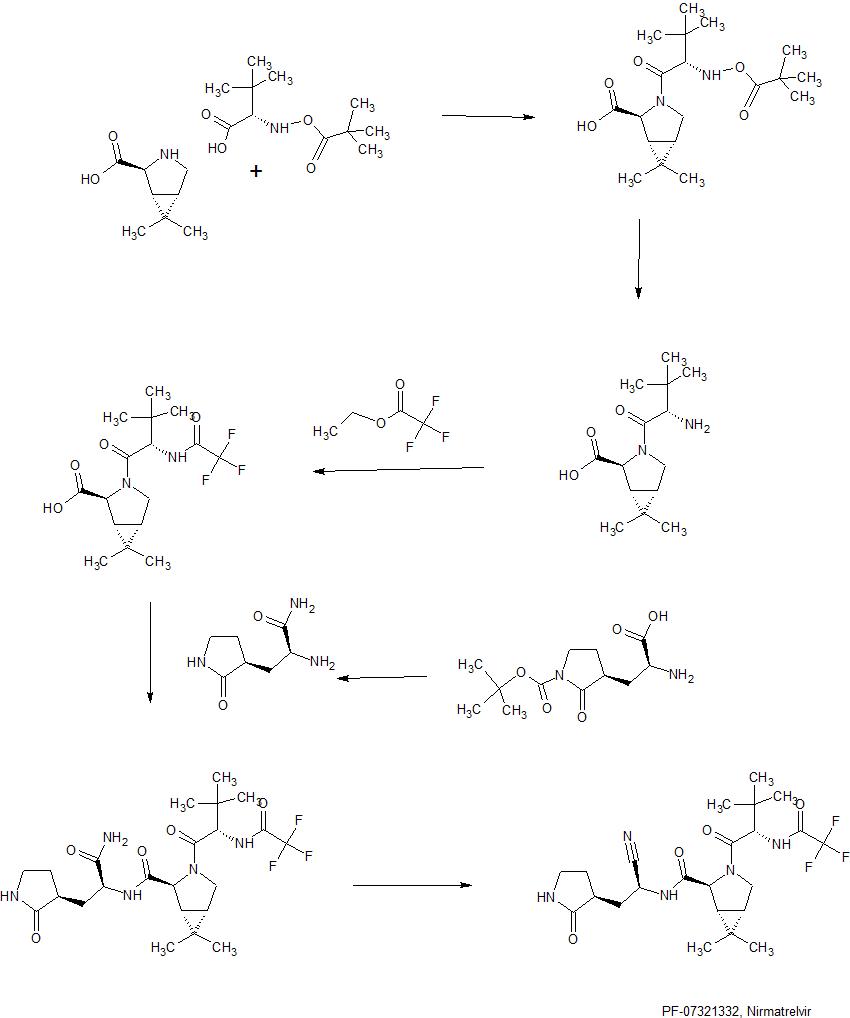
Synthesis of PF-07321332 (Compound 6): Anhydrous, MTBE solvate form
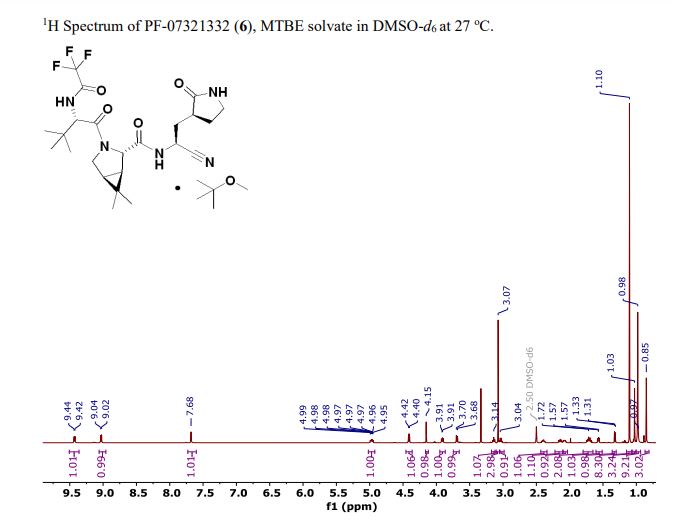
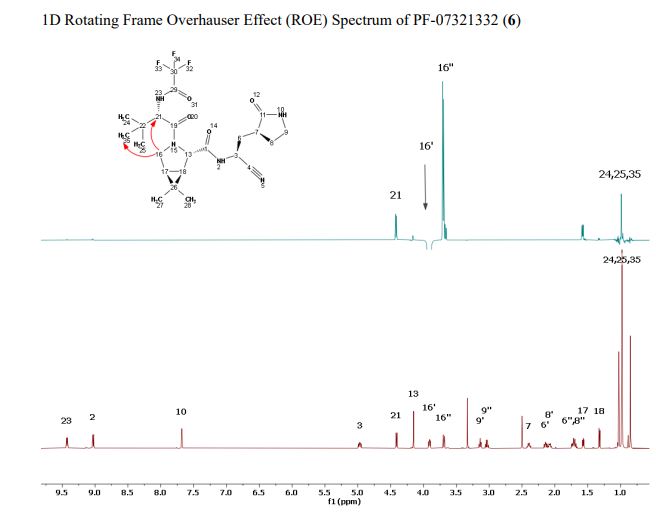
(1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin-3-yl]ethyl}-6,6-dimethyl-3-[3-methyl-N- (trifluoroacetyl)-L-valyl]-3-azabicyclo[3.1.0]hexane-2-carboxamide (1 eq tert-butyl methyl ether solvate) (6, MTBE solvate). This experiment was carried out in 2 parallel batches. Methyl N- (triethylammoniosulfonyl)carbamate, inner salt (Burgess reagent; 69.3 g, 276 mmol) was added to a solution of T18 (61 g, 111 mmol) in dichloromethane (550 ml). After the reaction mixture had been stirred at 25 °C for 1 h. The reaction mixture was quenched by a mixture of saturated aqueous sodium bicarbonate solution (200 ml) and saturated aqueous sodium chloride solution (100 ml). The separated organic phase was concentrated. The resulting residue was dissolved in 50% ethyl acetate/ tert-butyl methyl ether (600 ml), washed by a mixture of saturated aqueous sodium bicarbonate solution (200 ml) and saturated aqueous sodium chloride solution (100 ml) twice, saturated aqueous sodium chloride solution (200 ml), a mixture of HCl (1 M; 200 ml) and saturated aqueous sodium chloride solution (100 ml) twice. The organic layer was then dried over magnesium sulfate, filtered, and concentrated. The residue was treated with a mixture of ethyl acetate and tert-butyl methyl ether (1:10, 400 ml) and heated to 50 °C; after stirring for 1 hour at 50 °C, it was cooled to 25 °C and stirred overnight. The solid was collected via filtration, dissolved in dichloromethane (100 ml) and filtered through silica gel (200 g); the silica gel was then washed with ethyl acetate (1 Liter), 10% methanol in ethyl acetate (2 Liters). The combined eluates were concentrated. The 2 batches were combined, taken up in a mixture of ethyl acetate and tert-butyl methyl ether (5:95, 550 ml). This mixture was heated to 50 °C for 1 h, cooled to 25 °C, and stirred overnight. Filtration afforded 6, MTBE solvate, as a white solid. Yield: 104 g, 75 %. 1H NMR (600 MHz, DMSO-d6) δ 9.43 (d, J = 8.4 Hz, 1H), 9.03 (d, J = 8.6 Hz, 1H), 7.68 (s, 1H), 4.97 (ddd, J = 10.9, 8.6, 5.1 Hz, 1H), 4.41 (d, J = 8.4 Hz, 1H), 4.15 (s, 1H), 3.91 (dd, J = 10.4, 5.5 Hz, 1H), 3.69 (d, J = 10.4 Hz, 1H), 3.17 – 3.11 (m, 1H), 3.07 (s, 3H, MTBE), 3.04 (td, J = 9.4, 7.1 Hz, 1H), 2.40 (tdd, J = 10.4, 8.4, 4.4 Hz, 1H), 2.14 (ddd, J = 13.4, 10.9, 4.4 Hz,
1H), 2.11 – 2.03 (m, 1H), 1.76 – 1.65 (m, 2H), 1.57 (dd, J = 7.6, 5.5 Hz, 1H), 1.32 (d, J = 7.6 Hz, 1H), 1.10 (s, 9H, MTBE), 1.03 (s, 3H), 0.98 (s, 9H), 0.85 (s, 3H). Anal. Calcd for C23H32F3N5O4 .C5H12O: C, 57.23; H, 7.55; N, 11.92. Found: C, 57.08; H, 7.55; N, 11.85. mp = 118.8 oC
cry
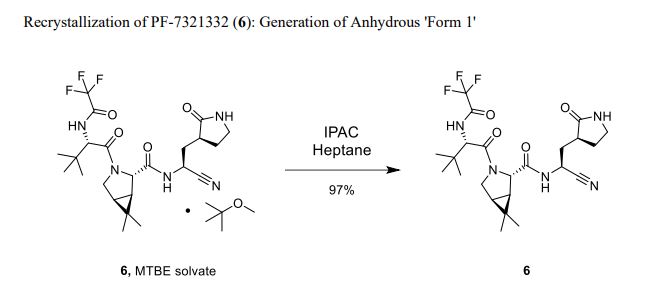
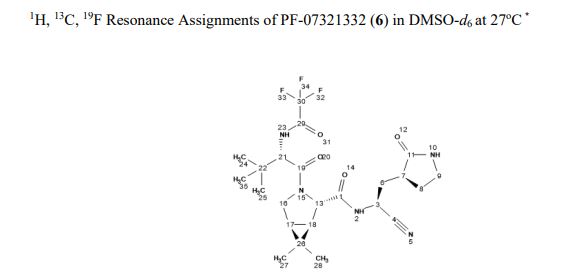
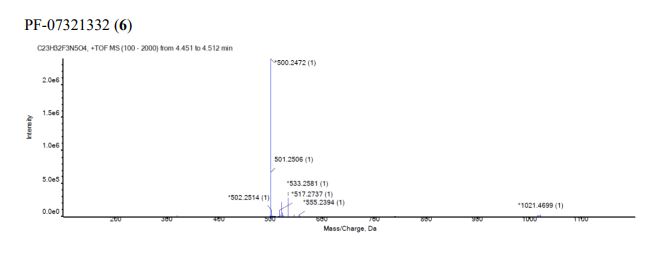
Compound 6 (anhydrous MTBE solvate, 200 g, 332.8 mmol, 83.11 mass%) was charged into a reactor with overhead half-moon stirring at 350 rpm. Heptane (1000 ml) was charged, followed by isopropyl acetate (1000 ml) and the stirring was continued at 20 oC overnight. Additional heptane (1000 ml) was charged over 120 minutes. The reaction vessel was then cooled to 10 oC over 30 min and stirred at that temp for 3 days. The solid was filtered, washing with a mixture of isopropyl acetate (80 ml) and heptane (320 ml). It was then dried under vacuum at 50 °C to provide 6, anhydrous ‘Form 1’, as a white crystalline solid. Yield: 160.93 g, 322 mmol, 97%. 1H NMR (600 MHz, DMSO-d6) δ 9.43 (d, J = 8.4 Hz, 1H), 9.03 (d, J = 8.6 Hz, 1H), 7.68 (s, 1H), 4.97 (ddd, J = 10.9, 8.6, 5.1 Hz, 1H), 4.41 (d, J = 8.4 Hz, 1H), 4.15 (s, 1H), 3.91 (dd, J = 10.4, 5.5 Hz, 1H), 3.69 (d, J = 10.4 Hz, 1H), 3.17 – 3.11 (m, 1H), 3.04 (td, J = 9.4, 7.1 Hz, 1H), 2.40 (tdd, J = 10.4, 8.4, 4.4 Hz, 1H), 2.14 (ddd, J = 13.4, 10.9, 4.4 Hz, 1H), 2.11 – 2.03 (m, 1H), 1.76 – 1.65 (m, 2H), 1.57 (dd, J = 7.6, 5.5 Hz, 1H), 1.32 (d, J = 7.6 Hz, 1H), 1.03 (s, 3H), 0.98 (s, 9H), 0.85 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 177.50, 170.72, 167.45, 156.95 (q, J = 37.0 Hz), 119.65, 115.84 (q, J = 286.9 Hz), 60.08, 58.19, 47.63, 37.77, 36.72, 34.60, 34.15, 30.28, 27.34, 26.86, 26.26, 25.72, 18.86, 12.34. 19F NMR (376 MHz, DMSO-d6) δ -72.94. HRMS (ESI-TOF) m/z calcd. for C23H33F3N5O4 [M + H]+ 500.2474, found 500.2472. Anal. Calcd for C23H32F3N5O4: C, 55.30; H, 6.46; N, 14.02. Found: C, 55.30; H, 6.49; N, 13.96. mp = 192.9 oC
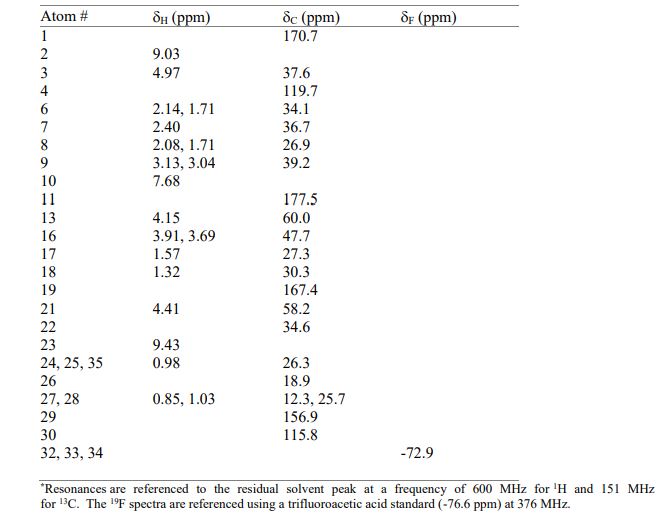
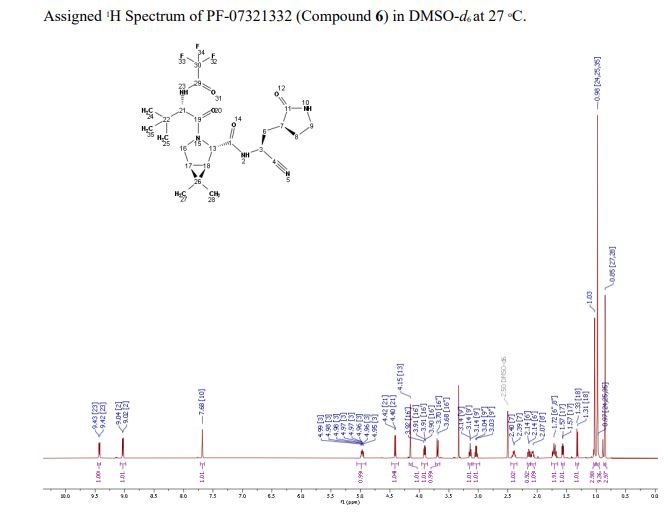
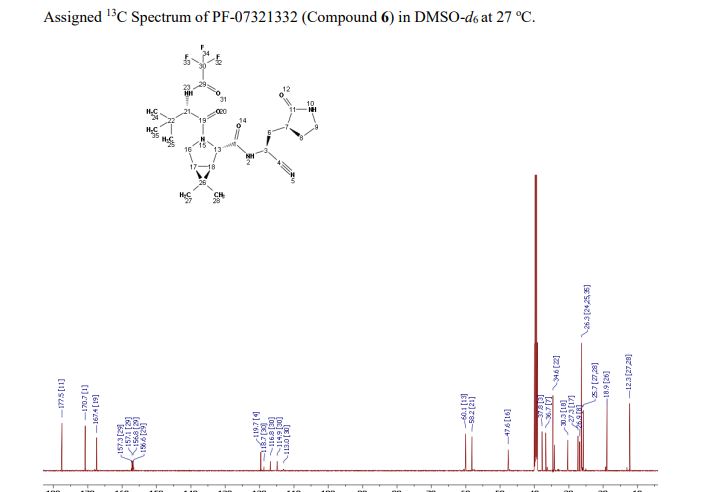
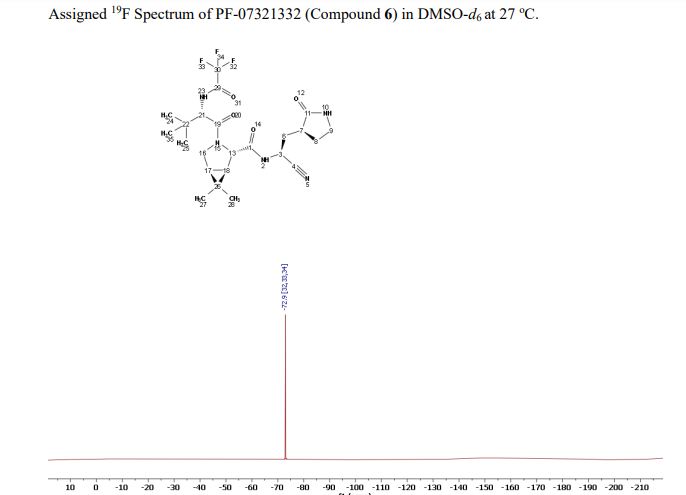
SYN
Bioorganic & medicinal chemistry letters (2021), 50, 128333.
https://www.sciencedirect.com/science/article/pii/S0960894X21005606
https://pubmed.ncbi.nlm.nih.gov/34418570/
pecific anti-coronaviral drugs complementing available vaccines are urgently needed to fight the COVID-19 pandemic. Given its high conservation across the betacoronavirus genus and dissimilarity to human proteases, the SARS-CoV-2 main protease (Mpro) is an attractive drug target. SARS-CoV-2 Mpro inhibitors have been developed at unprecedented speed, most of them being substrate-derived peptidomimetics with cysteine-modifying warheads. In this study, Mpro has proven resistant towards the identification of high-affinity short substrate-derived peptides and peptidomimetics without warheads. 20 cyclic and linear substrate analogues bearing natural and unnatural residues, which were predicted by computational modelling to bind with high affinity and designed to establish structure-activity relationships, displayed no inhibitory activity at concentrations as high as 100 μM. Only a long linear peptide covering residues P6 to P5‘ displayed moderate inhibition (Ki = 57 µM). Our detailed findings will inform current and future drug discovery campaigns targeting Mpro.
SYN
https://pubmed.ncbi.nlm.nih.gov/34498651/
Chemical communications (Cambridge, England) (2021), 57(72), 9096-9099We present a detailed computational analysis of the binding mode and reactivity of the novel oral inhibitor PF-07321332 developed against the SARS-CoV-2 3CL protease. Alchemical free energy calculations suggest that positions P3 and P4 could be susceptible to improvement in order to get a larger binding strength. QM/MM simulations unveil the reaction mechanism for covalent inhibition, showing that the nitrile warhead facilitates the recruitment of a water molecule for the proton transfer step.
PATENT
WO 2021234668
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021234668&_cid=P20-KX8D84-32732-1
SYN
In about November to December 2019 a novel coronavirus was identified as the cause of pneumonia cases in Wuhan (China). It spread, resulting in an epidemic throughout China, and thereafter in other countries throughout the world. In February 2020, the World Health Organization designated the disease COVTD-19, which stands for coronavirus disease 2019. The virus is also known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1)
COVID-19 is a betacoronavirus in the same subgenus as the severe acute respiratory syndrome (SARS) virus (as well as several bat coronaviruses), but in a different clade. The structure of the receptor- binding gene region is very similar to that of the SARS coronavirus, and the virus has been shown to use the same receptor, the angiotensin converting enzyme 2 (ACE2), for cell entry (2).
In the situation of rapidly increasing cases, inappropriate management of mild cases could increase the burden of healthcare system and medical costs. Viral clearance is a major standard in the assessment of recovery and discharge from medical care, but early results illustrated that the persistence of viral RNA is heterogeneous despite the rapid remission of symptoms and can last over three weeks even in very mild cases. In addition, long hospitalization stays may increase the risk for hospital-associated mental health problems and unexpected hospital-acquired infections. (9)
At the beginning, the outbreak identified an initial association with a seafood market that sold live animals in Wuhan, China. However, as the outbreak progressed, person-to-person spread became the main mode of transmission.
Person to person transmission is thought to occur mainly via respiratory droplets, resembling the spread of influenza. With droplet transmission, the virus is released in respiratory secretions when an infected person breathes, coughs, sneezes, or talks, and can infect another person if such secretions make direct contact with the mucous membranes. Infection can also occur if a person touches an infected surface and then touches his or her eyes, nose, or mouth. Droplets typically do not travel more than six feet (about two meters) and do not linger in the air. There is still controversy about this topic.
Whether SARS-CoV-2 can be transmitted through the airborne route (through particles smaller than droplets that remain in the air over time and distance) under natural conditions has been controversial.
Reflecting the current uncertainty regarding transmission mechanisms, recommendations on airborne precautions in the health care setting vary by location; airborne precautions are universally recommended when aerosol-generating procedures are performed.
It appears that SARS-CoV-2 can be transmitted prior to the development of symptoms and throughout the course of illness. However, most data informing this issue is from studies evaluating viral RNA detection from respiratory and other specimens, and detection of viral RNA does not necessarily indicate the presence of infectious virus.
A study suggested infectiousness started 2.3 days prior to symptom onset, peaked 0.7 days before symptom onset, and declined within seven days; however, most patients were isolated following symptom onset, which would reduce the risk of transmission later in illness regardless of infectiousness. These findings raise the possibility that patients might be more infectious in the earlier stage of infection, but additional data is needed to confirm this hypothesis (3).
How long a person remains infectious is also uncertain. The duration of viral shedding is variable; there appears to be a wide range, which may depend on severity of the illness. In one study of 21 patients with mild illness (no hypoxia), 90 percent had repeated negative viral RNA tests on nasopharyngeal swabs by 10 days after the onset of symptoms; tests were positive for longer in patients with more severe illness (4). In contrast, in another study of 56 patients with mild to moderate illness (none required intensive care), the median duration of viral RNA shedding from nasal or oropharyngeal specimens was 24 days, and the longest was 42 days (5). However, as mentioned above, detectable viral RNA does not always correlate with isolation of infectious virus, and there may be a threshold of viral RNA level below which infectivity is unlikely. In the study of nine patients with mild COVID-19 described above, infectious virus was not detected from respiratory specimens when the viral RNA level was <106 copies/mL (6).
Risk of transmission from an individual with SARS-CoV-2 infection varies by the type and duration of exposure, use of preventive measures, and likely individual factors (e.g., the amount of virus in respiratory secretions).
Antibodies against the virus are induced in those who have become infected. Preliminary evidence suggests that some of these antibodies are protective, but this remains to be definitively established. It is unknown whether all infected patients develop a protective immune response and how long any protective effect will last.
Diagnosis of COVID-19 is made by detection of SARS-CoV-2 RNA by reverse transcription polymerase chain reaction (RT-PCR). Various RT-PCR assays are used around the world; different assays amplify and detect different regions of the SARSCoV-2 genome. Common gene targets include nucleocapsid (N), envelope (E), spike (S), and RNA-dependent RNA polymerase (RdRp), as well as regions in the first open reading frame (7).
Serologic tests detect antibodies to SARS-CoV-2 in the blood, and those that have been adequately validated can help identify patients who have had COVID-19. However, sensitivity and specificity are still not well defined. Detectable antibodies generally take several days to weeks to develop, for example, up to 12 days for IgM and 14 days for IgG(Si-
………………………………..
PF-07321332 (or nirmatrelvir) is an antiviral drug developed by Pfizer which acts as an orally active 3CLprotease inhibitor. The combination of PF-07321332 with ritonavir has been in phase III trials for the treatment of COVID-19 since September 2021[2][3][4] and is expected to be sold under the brand name Paxlovid.[5] After promising results preventing hospitalization and death if given within the first 3 days of symptoms, Pfizer submitted an application to the U.S. Food and Drug Administration (FDA) for emergency authorization for PF-07321332 in combination with ritonavir in November 2021.[6]
PF-07321332 is an azabicyclohexane that is (1R,5S)-3-azabicyclo[3.1.0]hexane substituted by {(1S)-1-cyano-2-[(3S)-2-oxopyrrolidin-3-yl]ethyl}aminoacyl, 3-methyl-N-(trifluoroacetyl)-L-valinamide, methyl and methyl groups at positions 2S, 3, 6 and 6, respectively. It is an inhibitor of SARS-CoV-2 main protease which is currently under clinical development for the treatment of COVID-19. It has a role as an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor and an anticoronaviral agent. It is a nitrile, a member of pyrrolidin-2-ones, a secondary carboxamide, a pyrrolidinecarboxamide, a tertiary carboxamide, an organofluorine compound and an azabicyclohexane.
Development
Pharmaceutical
Coronaviral proteases cleave multiple sites in the viral polyprotein, usually after glutamine residues. Early work on related human rhinoviruses showed that the flexible glutamine side chain could be replaced by a rigid pyrrolidone.[7][8] These drugs had been further developed prior to the SARS CoV2 pandemic for other diseases including SARS.[9] The utility of targeting the 3CL protease in a real world setting was first demonstrated in 2018 when GC376 (a prodrug of GC373) was used to treat the previously 100% lethal cat coronavirus disease, feline infectious peritonitis, caused by Feline coronavirus.[10]
The Pfizer drug is an analog of GC373, where the aldehyde covalent cysteine acceptor has been replaced by a nitrile.[11][12]
PF-07321332 was developed by modification of an earlier clinical candidate lufotrelvir,[13][14] which is also a covalent inhibitor but its warhead is a phosphate prodrug of a hydroxyketone. However, lufotrelvir needs to be administered intravenously limiting its use to a hospital setting. Stepwise modification of the tripeptide protein mimetic led to PF-0732133, which is suitable for oral administration.[1] Key changes include a reduction in the number of hydrogen bond donors, and the number of rotatable bonds by introducing the rigid bicyclic non-canonical amino acid, which mimics the leucine residue found in earlier inhibitors. This residue had previously been used in the synthesis of boceprevir.[15]
Clinical
In April 2021, Pfizer began phase I trials.[16] In September 2021, Pfizer began a phase II/III trial.[17] In November 2021, Pfizer announced 89% reduction in hospitalizations of high risk patients studied when given within three days after symptom onset.[5]
On December 14, Pfizer announced that Paxlovid, when given within three days of symptom onset, reduced risk of hospitalization or death by 89% compared to placebo in 2,246 high risk patients studied.[18]
Chemistry and pharmacology
Full details of the synthesis of PF-07321332 were first published by scientists from Pfizer.[1]
In the penultimate step, a synthetic homochiral amino acid is coupled with a homochiral amino amide using the water-soluble carbodiimide EDCI as coupling agent. The resulting intermediate is then treated with Burgess reagent, which dehydrates the amide group to the nitrile of the product.
PF-07321332 is a covalent inhibitor, binding directly to the catalytic cysteine (Cys145) residue of the cysteine protease enzyme.[19]
In the drug combination, ritonavir serves to slow down metabolism of PF-07321332 by cytochrome enzymes to maintain higher circulating concentrations of the main drug.[20]
Public health system reactions to development
Despite not being approved yet in any country, the UK placed an order for 250,000 courses after Pfizer´s press release in October 2021,[21][22] and Australia pre-ordered 500,000 courses of the drug.[23]
As of November 2021, the US government was expected to sign a contract to buy around 10 million courses of the combination treatment.[24][25]
Legal status
In November 2021, Pfizer signed a license agreement with the United Nations–backed Medicines Patent Pool to allow PF-07321332 to be manufactured and sold in 95 countries.[26] Pfizer stated that the agreement will allow local medicine manufacturers to produce the pill “with the goal of facilitating greater access to the global population”. However, the deal excludes several countries with major COVID-19 outbreaks including Brazil, China, Russia, Argentina, and Thailand.[27][28]
On 16 November 2021, Pfizer submitted an application to the U.S. Food and Drug Administration (FDA) for emergency authorization for PF-07321332 in combination with ritonavir.[29][30][31]
Misleading comparison with ivermectin
Conspiracy theorists on the internet have claimed that Paxlovid is merely a “repackaged” version of the antiparasitic drug ivermectin, which has been erroneously promoted as a COVID-19 “miracle cure”. Their claims, sometimes using the nickname “Pfizermectin”,[32] are based on a narrative that Pfizer is suppressing the true benefits of ivermectin and rely on superficial correspondences between the drugs and a misunderstanding of their respective pharmacokinetics.[33] Paxlovid is not structurally related or similar to ivermectin, and while both are 3C-like protease inhibitors, Paxlovid is much more potent with an IC50 around 10,000 times lower, allowing for effective oral dosing within the therapeutic margin.[34]
References
- ^ Jump up to:a b c Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. (November 2021). “An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19″. Science: eabl4784. doi:10.1126/science.abl4784. PMID 34726479. S2CID 240422219.
- ^ Vandyck K, Deval J (August 2021). “Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection”. Current Opinion in Virology. 49: 36–40. doi:10.1016/j.coviro.2021.04.006. PMC 8075814. PMID 34029993.
- ^ Şimşek-Yavuz S, Komsuoğlu Çelikyurt FI (August 2021). “Antiviral treatment of COVID-19: An update”. Turkish Journal of Medical Sciences. doi:10.3906/sag-2106-250. PMID 34391321. S2CID 237054672.
- ^ Ahmad B, Batool M, Ain QU, Kim MS, Choi S (August 2021). “Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations”. International Journal of Molecular Sciences. 22 (17): 9124. doi:10.3390/ijms22179124. PMC 8430524. PMID 34502033.
- ^ Jump up to:a b “Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk Of Hospitalization Or Death By 89% In Interim Analysis Of Phase 2/3 EPIC-HR Study”. Pfizer Inc. 5 November 2021.
- ^ Mahase E (November 2021). “Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports”. BMJ. 375: n2713. doi:10.1136/bmj.n2713. PMID 34750163. S2CID 243834203.
- ^ Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R (June 2003). “Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs”. Science. 300 (5626): 1763–1767. Bibcode:2003Sci…300.1763A. doi:10.1126/science.1085658. PMID 12746549. S2CID 13031405.
- ^ Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, et al. (April 1999). “Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements”. Journal of Medicinal Chemistry. 42 (7): 1213–1224. doi:10.1021/jm9805384. PMID 10197965.
- ^ Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH (July 2016). “An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy”. Journal of Medicinal Chemistry. 59 (14): 6595–6628. doi:10.1021/acs.jmedchem.5b01461. PMC 7075650. PMID 26878082.
- ^ Pedersen NC, Kim Y, Liu H, Galasiti Kankanamalage AC, Eckstrand C, Groutas WC, et al. (April 2018). “Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis”. Journal of Feline Medicine and Surgery. 20 (4): 378–392. doi:10.1177/1098612X17729626. PMC 5871025. PMID 28901812.
- ^ Halford B (7 April 2021). “Pfizer unveils its oral SARS-CoV-2 inhibitor”. Chemical & Engineering News. 99 (13): 7. doi:10.47287/cen-09913-scicon3. S2CID 234887434.
- ^ Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, et al. (August 2020). “Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication”. Nature Communications. 11 (1): 4282. doi:10.1038/s41467-020-18096-2. PMC 7453019. PMID 32855413.
- ^ Clinical trial number NCT04535167 for “First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19 ” at ClinicalTrials.gov
- ^ Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, et al. (February 2021). “Discovery of a Novel Inhibitor of Coronavirus 3CL Protease for the Potential Treatment of COVID-19”. bioRxiv: 2020.09.12.293498. doi:10.1101/2020.09.12.293498. PMC 7491518. PMID 32935104.
- ^ Njoroge FG, Chen KX, Shih NY, Piwinski JJ (January 2008). “Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection”. Accounts of Chemical Research. 41 (1): 50–59. doi:10.1021/ar700109k. PMID 18193821. S2CID 2629035.
- ^ Nuki P (26 April 2021). “Pfizer is testing a pill that, if successful, could become first-ever home cure for COVID-19”. National Post. Archived from the original on 27 April 2021.
- ^ “Pfizer begins dosing in Phase II/III trial of antiviral drug for Covid-19”. Clinical Trials Arena. 2 September 2021.
- ^ Press release (14 December 2021). “Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death”.
- ^ Pavan M, Bolcato G, Bassani D, Sturlese M, Moro S (December 2021). “Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332”. J Enzyme Inhib Med Chem. 36 (1): 1646–1650. doi:10.1080/14756366.2021.1954919. PMC 8300928. PMID 34289752.
- ^ Woodley M (19 October 2021). “What is Australia’s potential new COVID treatment?”. The Royal Australian College of General Practitioners (RACGP). Retrieved 6 November 2021.
- ^ “Pfizer Covid pill ‘can cut hospitalisations and deaths by nearly 90%'”. The Guardian. 5 November 2021. Retrieved 17 November 2021.
- ^ Mahase E (October 2021). “Covid-19: UK stockpiles two unapproved antiviral drugs for treatment at home”. BMJ. 375: n2602. doi:10.1136/bmj.n2602. PMID 34697079. S2CID 239770104.
- ^ “What are the two new COVID-19 treatments Australia has gained access to?”. ABC News (Australia). 17 October 2021. Retrieved 5 November 2021.
- ^ “U.S. to Buy Enough of Pfizer’s Covid Antiviral Pills for 10 Million People”. The New York Times. 17 November 2021. Retrieved 17 November 2021.
- ^ Pager T, McGinley L, Johnson CY, Taylor A, Parker C. “Biden administration to buy Pfizer antiviral pills for 10 million people, hoping to transform pandemic”. The Washington Post. Retrieved 16 November 2021.
- ^ “Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries” (Press release). Pfizer. 16 November 2021. Retrieved 17 November 2021 – via Business Wire.
- ^ “Covid-19: Pfizer to allow developing nations to make its treatment pill”. BBC News. 16 November 2021. Archived from the original on 16 November 2021. Retrieved 17 November 2021.
- ^ “Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries”. The New York Times. 16 November 2021. Retrieved 17 November 2021.
- ^ “Pfizer Seeks Emergency Use Authorization for Novel COVID-19 Oral Antiviral Candidate”. Business Wire (Press release). 16 November 2021. Retrieved 17 November 2021.
- ^ Kimball S (16 November 2021). “Pfizer submits FDA application for emergency approval of Covid treatment pill”. CNBC. Retrieved 17 November 2021.
- ^ Robbins R (5 November 2021). “Pfizer Says Its Antiviral Pill Is Highly Effective in Treating Covid”. The New York Times. ISSN 0362-4331. Archived from the original on 8 November 2021. Retrieved 9 November 2021.
- ^ Bloom J (2 December 2021). “How Does Pfizer’s Pavloxid Compare With Ivermectin?”. American Council on Science and Health. Retrieved 12 December 2021.
- ^ Gorski D (15 November 2021). “Pfizer’s new COVID-19 protease inhibitor drug is not just ‘repackaged ivermectin'”. Science-Based Medicine.
- ^ von Csefalvay C (27 November 2021). “Why Paxlovid is not Pfizermectin”. Bits and Bugs. Chris von Csefalvay. Retrieved 28 November 2021.
External links
- “PF-07321332”. Drug Information Portal. U.S. National Library of Medicine.
- “Early Data Suggest Pfizer Pill May Prevent Severe COVID-19”. National Institutes of Health. 16 November 2021.
Pfizer to make COVID-19 pill available in low- and middle-income nations
If authorized by global health authorities, the drug promises to reduce deaths and hospitalizations linked to
the novel coronavirus.
By Brian Buntz | November 16, 2021FacebookTwitterLinkedInShare
In late October, Merck (NYSE:MRK) and its partner Ridgeback Biotherapeutics agreed to make the COVID-19 antiviral molnupiravir available in the developing world.
Now, Pfizer (NYSE:PFE) is taking a similar approach for its investigational antiviral cocktail Paxlovid, which contains PF-07321332 and ritonavir.
Pfizer, like Merck, struck an agreement with the Medicines Patent Pool (MPP) related to Paxlovid.
MPP’s mission is to expand low- and middle-income countries’ access to vital medicines. The United Nations supports the organization.
Pfizer announced earlier this month that Paxlovid was 89% effective in reducing the risk of hospitalization or death in an interim analysis of the Phase 2/3 EPIC-HR trial.
The collaboration with MPP will enable generic drug makers internationally with sub-licenses to produce Paxlovid for use in 95 countries, which comprise more than half of the world’s population.
“This license is so important because, if authorized or approved, this oral drug is particularly well-suited for low- and middle-income countries and could play a critical role in saving lives, contributing to global efforts to fight the current pandemic,” said Charles Gore, executive director of MPP, in a press release. “PF-07321332 is to be taken together with ritonavir, an HIV medicine we know well, as we have had a license on it for many years, and we will be working with generic companies to ensure there is enough supply for both COVID-19 and HIV.”
At present, MPP has signed agreements with ten patient holders for 13 HIV antiretrovirals and several other drugs.
Filed Under: clinical trials, Drug Discovery, Infectious Disease
Tagged With: Medicines Patent Pool, Merck, PF-07321332, Pfizer, Ridgeback Biotherapeutics, ritonavir
PFIZER INITIATES PHASE 1 STUDY OF NOVEL ORAL ANTIVIRAL THERAPEUTIC AGENT AGAINST SARS-COV-2
Tuesday, March 23, 2021 – 11:00am
- In-vitro studies conducted to date show that the clinical candidate PF-07321332 is a potent protease inhibitor with potent anti-viral activity against SARS-CoV-2
- This is the first orally administered coronavirus-specific investigational protease inhibitor to be evaluated in clinical studies, and follows Pfizer’s intravenously administered investigational protease inhibitor, which is currently being evaluated in a Phase 1b multi-dose study in hospitalized clinical trial participants with COVID-19
NEW YORK–(BUSINESS WIRE)– Pfizer Inc. (NYSE: PFE) announced today that it is progressing to multiple ascending doses after completing the dosing of single ascending doses in a Phase 1 study in healthy adults to evaluate the safety and tolerability of an investigational, novel oral antiviral therapeutic for SARS-CoV-2, the virus that causes COVID-19. This Phase 1 trial is being conducted in the United States. The oral antiviral clinical candidate PF-07321332, a SARS-CoV2-3CL protease inhibitor, has demonstrated potent in vitro anti-viral activity against SARS-CoV-2, as well as activity against other coronaviruses, suggesting potential for use in the treatment of COVID-19 as well as potential use to address future coronavirus threats.
“Tackling the COVID-19 pandemic requires both prevention via vaccine and targeted treatment for those who contract the virus. Given the way that SARS-CoV-2 is mutating and the continued global impact of COVID-19, it appears likely that it will be critical to have access to therapeutic options both now and beyond the pandemic,” said Mikael Dolsten, MD, PhD., Chief Scientific Officer and President, Worldwide Research, Development and Medical of Pfizer. “We have designed PF-07321332 as a potential oral therapy that could be prescribed at the first sign of infection, without requiring that patients are hospitalized or in critical care. At the same time, Pfizer’s intravenous antiviral candidate is a potential novel treatment option for hospitalized patients. Together, the two have the potential to create an end to end treatment paradigm that complements vaccination in cases where disease still occurs.”
Protease inhibitors bind to a viral enzyme (called a protease), preventing the virus from replicating in the cell. Protease inhibitors have been effective at treating other viral pathogens such as HIV and hepatitis C virus, both alone and in combination with other antivirals. Currently marketed therapeutics that target viral proteases are not generally associated with toxicity and as such, this class of molecules may potentially provide well-tolerated treatments against COVID-19.
The Phase 1 trial is a randomized, double-blind, sponsor-open, placebo-controlled, single- and multiple-dose escalation study in healthy adults evaluating the safety, tolerability and pharmacokinetics of PF-07321332.
Initiation of this study is supported by preclinical studies that demonstrated the antiviral activity of this potential first-in-class SARS-CoV-2 therapeutic designed specifically to inhibit replication of the SARS-CoV2 virus. The structure of PF-07321332, together with the pre-clinical data, will be shared in a COVID-19 session of the Spring American Chemical Society meeting on April 6.
Pfizer is also investigating an intravenously administered investigational protease inhibitor, PF-07304814, which is currently in a Phase 1b multi-dose trial in hospitalized clinical trial participants with COVID-19.
About Pfizer: Breakthroughs That Change Patients’ Lives
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products, including innovative medicines and vaccines. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world’s premier innovative biopharmaceutical companies, we collaborate with health care providers, governments and local communities to support and expand access to reliable, affordable health care around the world. For more than 170 years, we have worked to make a difference for all who rely on us. We routinely post information that may be important to investors on our website at www.Pfizer.com. In addition, to learn more, please visit us on www.Pfizer.com and follow us on Twitter at @Pfizer and @Pfizer News, LinkedIn, YouTube and like us on Facebook at Facebook.com/Pfizer.
.CLIP
https://cen.acs.org/content/cen/articles/99/i13/Pfizer-unveils-oral-SARS-CoV.html

Drugmaker Pfizer revealed its oral COVID-19 antiviral clinical candidate PF-07321332 on Tuesday at the American Chemical Society Spring 2021 meeting. The compound, which is currently in Phase 1 clinical trials, is the first orally administered compound in the clinic that targets the main protease (also called the 3CL protease) of SARS-CoV-2, the virus that causes COVID-19. By inhibiting the main protease, PF-07321332 prevents the virus from cleaving long protein chains into the parts it needs to reproduce itself. Dafydd Owen, director of medicinal chemistry at Pfizer, presented the compound in a symposium of the Division of Medicinal Chemistry.
Last year, Pfizer reported PF-07304814, a different small molecule inhibitor of SARS-CoV-2’s main protease. The work to develop that compound began during the 2002-2003 outbreak of SARS-CoV, severe acute respiratory syndrome. But that molecule can only be given intravenously, which limits its use to hospital settings.
Because PF-07321332 can be taken orally, as a pill or capsule, it could be given outside of hospitals if it proves to be safe and effective. People who have been exposed to SARS-CoV-2 could take it as a preventative measure, for example.
“For the foreseeable future, we will expect to see continued outbreaks from COVID-19. And therefore, as with all viral pandemics, it’s important we have a full toolbox on how to address it,” Charlotte Allerton, Pfizer’s head of medicine design, told C&EN.
PF-07321332 was developed from scratch during the current pandemic. It’s a reversible covalent inhibitor that reacts with one of the main protease’s cysteine residues. Owen also discussed the chemistry involved in scaling up the compound. The first 7 mg of the compound were synthesized in late July 2020. Encouraged by the early biological data, the Pfizer team aimed to scale up the synthesis. By late October, they’d made 100 g of the compound. Just two weeks later, the chemists had scaled up the synthesis to more than 1 kg. Owen said 210 researchers had worked on the project. Ana Martinez, who studies COVID-19 treatments at the Spanish National Research Council CSIC and also presented during the symposium, told C&EN that having a COVID-19 antiviral is of critical importance. She eagerly anticipates the safety and efficacy data from the trials of PF-07321332. “Hopefully we will have a new drug to fight against COVID-19,” Martinez said. And because the molecule targets the main protease, she said that it might be useful for fighting other coronaviruses and preventing future pandemics.Chemical & Engineering News
| Clinical data | |
|---|---|
| ATC code | None |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 2628280-40-8 |
| PubChem CID | 155903259 |
| UNII | 7R9A5P7H32 |
| KEGG | D12244 |
| ChEBI | CHEBI:170007 |
| Chemical and physical data | |
| Formula | C23H32F3N5O4 |
| Molar mass | 499.535 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| Melting point | 192.9[1] °C (379.2 °F) |
| showSMILES | |
| showInChI |
Xray crystal structure (PDB:7SI9 and 7VH8) of the SARS-CoV-2 protease inhibitor PF-07321332 bound to the viral 3CLpro (Mpro) protease enzyme. Ribbon diagram of the protein with the drug shown as sticks. The catalytic residues (His41, Cys145) are shown as yellow sticks.
./////////////////PF-07321332, PF 07321332, COVID 19, CORONA VIRUS, SARS-CoV-2 inhibitor, PHASE 1, nirmatrelvir, PAXLOVID, CORONA VIRUS, COVID 19
C1N(C([C@@H]2C1[C@]2(C)C)C(=O)N[C@@H](CC3C(NCC3)=O)C#N)C(C([C@@](C)(C)C)NC(=O)C(F)(F)F)=O
C1N(C(C2C1C2(C)C)C(=O)N[C@@H](CC3C(NCC3)=O)C#N)C(C([C@@](C)(C)C)NC(=O)C(F)(F)F)=O















