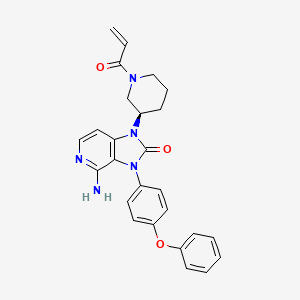
Tolebrutinib
SAR442168
-
Treatment of Multiple Sclerosis (MS)
CAS 1971920-73-6
PRN 2246, example 3 [WO2016196840A1]
C26H25N5O3,
| 455.5 |
4-amino-3-(4-phenoxyphenyl)-1-[(3R)-1-prop-2-enoylpiperidin-3-yl]imidazo[4,5-c]pyridin-2-one
4-amino-3-(4-phenoxyphenyl)-1-[(3R)-1-prop-2-enoylpiperidin-3-yl]imidazo[4,5-c]pyridin-2-one
(R)-1-(1-Acryloylpiperidin-3-yl)-4-amino-3-(4-phenoxyphenyl)-1H-imidazo[4,5-c]pyridin-2(3H)-one
4-amino-3-(4-phenoxyphenyl)-1-[(3R)-1-(prop-2-
enoyl)piperidin-3-yl]-1,3-dihydro-2H-imidazo[4,5-
Tolebrutinib (R&D code SAR442168), developed by Principia and later acquired by Sanofi and included in its product line, Tolebrutinib is a BTK inhibitor used to treat cancer, autoimmune diseases such as multiple sclerosis and myasthenia gravis, inflammatory diseases and thromboembolic diseases, etc.,
Tolebrutinib is an orally bioavailable, brain-penetrant, selective, small molecule inhibitor of Bruton’s tyrosine kinase (BTK), with potential immunomodulatory and anti-inflammatory activities. Upon oral administration, tolebrutinib is able to cross the blood-brain barrier and inhibits the activity of BTK both peripherally and in the central nervous system (CNS). This prevents the activation of the B-cell antigen receptor (BCR) signaling pathway, and the resulting immune activation and inflammation. The inhibition of BTK activity also prevents microglial inflammatory signaling in the CNS, and the resulting immune activation, neuroinflammation and neurodegeneration. BTK, a cytoplasmic tyrosine kinase and member of the Tec family of kinases, plays an important role in B lymphocyte development, activation, signaling, proliferation and survival. In addition to B cells, BTK is also expressed in innate immune cells, including macrophages and microglia, and plays an important role in the regulation of microglial inflammatory signaling.
BTK, a member of the Tec family non-receptor tyrosine kinases, is essential for B cell signaling downstream from the B-cell receptor. It is expressed in B cells and other hematopoietic cells such as monocytes, macrophages and mast cells. It functions in various aspects of B cell function that maintain the B cell repertoire (see Gauld S. B. et al., B cell antigen receptor signaling: roles in cell development and disease. Science,
296: 1641 -2. 2002.) B cells pay a role in rheumatoid arthritis (see Perosa F., et ai, CD20-depleting therapy in autoimmune diseases: from basic research to the clinic. / Intern Med. 267:260-77. 2010 and Dorner T, et at. Targeting B cells in immune-mediated
inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol The 125:464-75. 2010 and Honigberg, L., et. ai, The selective BTK inhibitor PCI-32765 blocks B cell and mast cell activation and prevents mouse collagen indiced arthritis. Clin. Immunol. 127 SI :S 111. 2008) and in other autoimmune diseases such as systemic lupus erythematosus and cancers (see Shlomchik M. J., et. ai, The role of B cells in lpr/lpr-induced autoimmunity. /. Exp Med. 180:1295-1306. 1994; Honigberg L. A., The Braton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. 107: 13075-80. 2010; and Mina-Osorio P, et al., Suppression of
glomerulonephritis in lupus-prone NZB x NZW mice by RN486, a selective inhibitor of Bruton’s tyrosine kinase. Arthritis Rheum. 65: 2380-91. 2013).
There is also potential for BTK inhibitors for treating allergic diseases (see Honigberg, L., et. al., The selective BTK inhibitor PCI-32765 blocks B cell and mast cell activation and prevents mouse collagen indiced arthritis. Clin. Immunol. 127 SI :S111. 2008). It was noted that the irreversible inhibitor suppresses passive cutaneous anaphylaxis (PCA) induced by IgE antigen complex in mice. These findings are in agreement with those noted with BTK-mutant mast cells and knockout mice and suggest that BTK inhibitors may be useful for the treatment of asthma, an IgE-dependent allergic disease of the airway.
Accordingly, compounds that inhibit BTK would be useful in treatment for diseases such as autoimmune diseases, inflammatory diseases, and cancer.
PATENT
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016196840
Example 3
Synthesis of (R)-l-(l-acryloylpiperidin-3-yl)-4-amino-3-(4-phenoxyphenyl)-lH- imidazo[4,5-c]pyridin-2(3H)-one
Into a 100-mL round-bottom flask, was placed (R)-4-amino-3-(4-phenoxyphenyl)-l-(piperidin-3-yl)-lH-imidazo[4,5-c]pyridin-2(3H)-one (150 mg, 0.37 mmol, 1.00 equiv), DCM-CH30H (6 mL), TEA (113 mg, 1.12 mmol, 3.00 equiv). This was followed by the addition of prop-2-enoyl chloride (40.1 mg, 0.44 mmol, 1.20 equiv) dropwise with stirring at OoC in 5 min. The resulting solution was stirred for 2 h at 0 °C. The resulting mixture was concentrated under vacuum. The residue was applied onto a silica gel column with dichloromethane/methanol (30: 1). The crude product (100 mg) was purified by Prep-HPLC with the following conditions (Column, XBridge Prep CI 8 OBD
Column,5um, 19*150mm; mobile phase, water with 0.05%TFA and ACN (25.0% ACN up to 45.0% in 8 min). 54.5 mg product of (R)-l-(l -acryloylpiperidin-3-yl)-4-amino-3-(4-phenoxyphenyl)-lH-imidazo[4,5-c]pyridin-2(3H)-one was obtained as a white solid. LC-MS m/z: 465.2 (M+l)
Step 2
Into a 25-mL round-bottom flask was placed tert-butyl (3R)-3-[4-[(E)-[(dimethy]amino)-methylidene]-amino]-2-oxo-3-(4-phenoxyphenyl)-lH,2H,3H-imidazo[4,5-c]pyridin-l -yl]piperidine- l-carboxylate (150 mg, 0.27 mmol, 1.00 equiv), 1,4-dioxane (6 mL), and hydrogen chloride (3 mL). The resulting solution was stirred overnight at 50° C. The reaction mixture was quenched with water. The pH of the solution was adjusted to 9 with sodium bicarbonate. The resulting solution was extracted with dichloromethane:CH3OH=10: 1 and the organic layers were combined. The resulting mixture was washed with sodium chloride and the organic layers were combined, dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column and eluted with dichloromethane/methanol (30: 1) to give 80 mg (74%) of 4-amino-3-(4-phenoxyphenyl)-l -[(3R)-piperidin-3-yl]-lH,2H,3H-imidazo[4,5-c]pyridin-2-one as a light yellow solid.
////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
/////////Tolebrutinib, SAR 442168, PRN 2246, GTPL10625, BTK’168, EX-A4699, BDBM50557487, WHO 11268, Multiple Sclerosis, (MS),