
Triheptanoin
Approved US FDA 30/6/2020 Dojolvi UX 007
Triheptanoin is a source of heptanoate fatty acids, which can be metabolized without the enzymes of long chain fatty acid oxidation.4 In clinical trials, patients with long chain fatty acid oxidation disorders (lc-FAODs) treated with triheptanoin are less likely to develop hypoglycemia, cardiomyopathy, rhabdomyolysis, and hepatomegaly.1,2 Complications in lc-FAOD patients are reduced from approximately 60% to approximately 10% with the addition of triheptanoin.2
Triheptanoin was granted FDA approval on 30 June 2020.4
Triheptanoin, sold under the brand name Dojolvi, is a medication for the treatment of children and adults with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD).[1][2][3]
The most common adverse reactions include abdominal pain, diarrhea, vomiting, and nausea.[1][2][3]
Triheptanoin was approved for medical use in the United States in June 2020.[4][2][3]
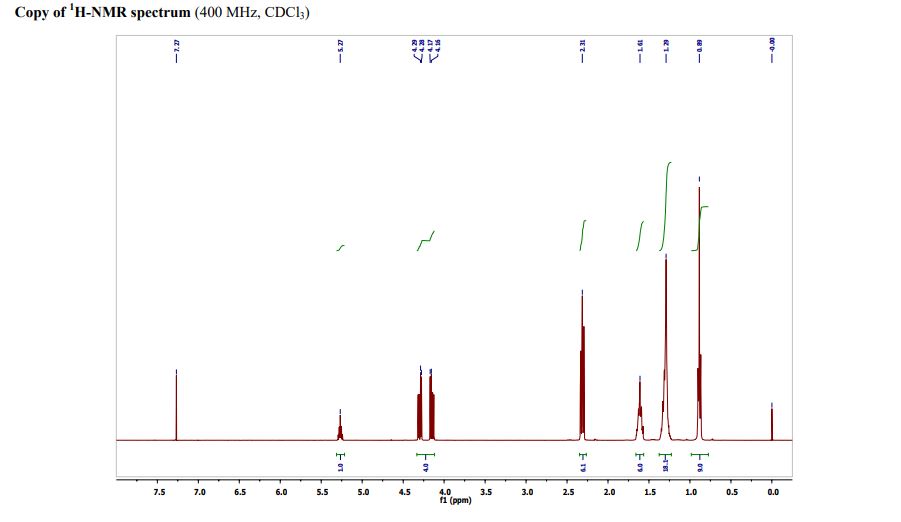
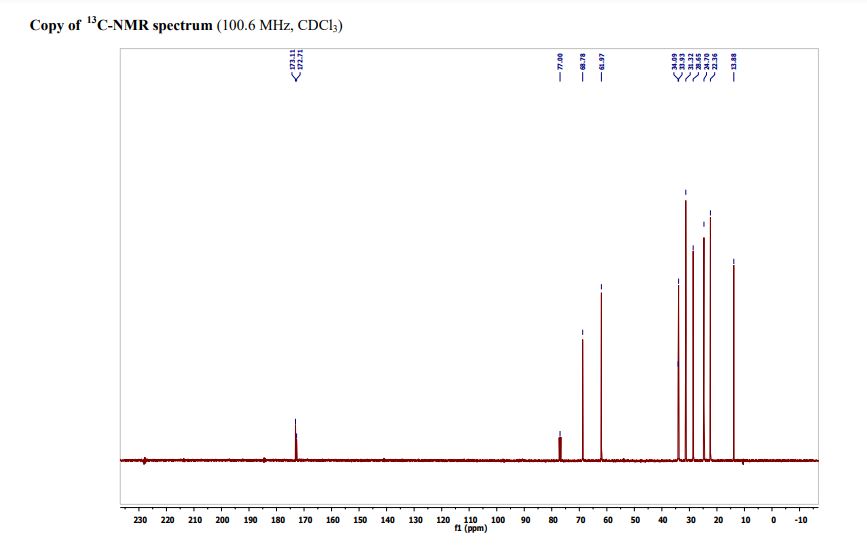
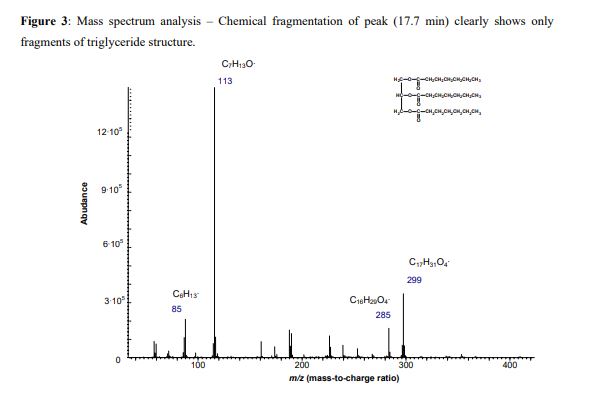
Triheptanoin is a triglyceride that is composed of three seven-carbon (C7:0) fatty acids. These odd-carbon fatty acids are able to provide anaplerotic substrates for the TCA cycle. Triheptanoin is used clinically in humans to treat inherited metabolic diseases, such as pyruvate carboxylase deficiency and carnitine palmitoyltransferase II deficiency. It also appears to increase the efficacy of the ketogenic diet as a treatment for epilepsy.
Since triheptanoin is composed of odd-carbon fatty acids, it can produce ketone bodies with five carbon atoms, as opposed to even-carbon fatty acids which are metabolized to ketone bodies with four carbon atoms. The five-carbon ketones produced from triheptanoin are beta-ketopentanoate and beta-hydroxypentanoate. Each of these ketone bodies easily crosses the blood–brain barrier and enters the brain.
Medical uses
Dojolvi is indicated as a source of calories and fatty acids for the treatment of children and adults with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD).[1][2]
History
Triheptanoin was designated an orphan drug by the U.S. Food and Drug Administration (FDA) in 2006, 2008, 2014, and 2015.[5][6][7][8] Triheptanoin was also designated an orphan drug by the European Medicines Agency (EMA).[9][10][11][12][13][14][15][16]
Triheptanoin was approved for medical use in the United States in June 2020.[4][2]
The FDA approved triheptanoin based on evidence from three clinical trials (Trial 1/NCT018863, Trial 2/NCT022141 and Trial 3/NCT01379625).[3] The trials enrolled children and adults with LC-FAOD.[3] Trials 1 and 2 were conducted at 11 sites in the United States and the United Kingdom, and Trial 3 was conducted at two sites in the United States.[3]
Trial 1 and Trial 2 were used to evaluate the side effects of triheptanoin.[3] Both trials enrolled children and adults diagnosed with LC-FAOD.[3] In Trial 1, participants received triheptanoin for 78 weeks.[3] Trial 2 enrolled participants from other trials who were already treated with triheptanoin (including those from Trial 1) as well as participants who were never treated with triheptanoin before.[3] Trial 2 is still ongoing and is planned to last up to five years.[3]
The benefit of triheptanoin was evaluated in Trial 3 which enrolled enrolled children and adults with LC-FAOD.[3] Half of the participants received triheptanoin and half received trioctanoin for four months.[3] Neither the participants nor the investigators knew which treatment was given until the end of the trial.[3] The benefit of triheptanoin in comparison to trioctanoin was assessed by measuring the changes in heart and muscle function.[3]
Names
Triheptanoin is the international nonproprietary name.[17]
SYN
https://onlinelibrary.wiley.com/doi/abs/10.1002/ejlt.201100425

References
- ^ Jump up to:a b c d “Dojolvi- triheptanoin liquid”. DailyMed. 30 June 2020. Retrieved 24 September2020.
- ^ Jump up to:a b c d e “Ultragenyx Announces U.S. FDA Approval of Dojolvi (UX007/triheptanoin), the First FDA-Approved Therapy for the Treatment of Long-chain Fatty Acid Oxidation Disorders”. Ultragenyx Pharmaceutical. 30 June 2020. Retrieved 30 June 2020 – via GlobeNewswire.
- ^ Jump up to:a b c d e f g h i j k l m n o “Drug Trials Snapshots: Dojolvi”. U.S. Food and Drug Administration. 30 June 2020. Retrieved 16 July 2020.
- ^ Jump up to:a b “Dojolvi: FDA-Approved Drugs”. U.S. Food and Drug Administration (FDA). Retrieved 30 June 2020.
- ^ “Triheptanoin Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). 26 May 2006. Retrieved 30 June 2020.
- ^ “Triheptanoin Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). 1 February 2008. Retrieved 30 June 2020.
- ^ “Triheptanoin Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). 21 October 2014. Retrieved 30 June 2020.
- ^ “Triheptanoin Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). 15 April 2015. Retrieved 30 June 2020.
- ^ “EU/3/12/1081”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/12/1082”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/15/1495”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/15/1508”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/15/1524”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/15/1525”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/15/1526”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ “EU/3/16/1710”. European Medicines Agency (EMA). Retrieved 30 June 2020.
- ^ World Health Organization (2019). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82”. WHO Drug Information. 33 (3): 694. hdl:10665/330879. License: CC BY-NC-SA 3.0 IGO.
Further reading
- de Almeida Rabello Oliveira M, da Rocha Ataíde T, de Oliveira SL, de Melo Lucena AL, de Lira CE, Soares AA, et al. (March 2008). “Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats”. Neurosci. Lett. 434 (1): 66–70. doi:10.1016/j.neulet.2008.01.032. PMID 18281154. S2CID 7754768.
- Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, Rabier D, et al. (April 2005). “Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy”. Mol. Genet. Metab. 84 (4): 305–12. doi:10.1016/j.ymgme.2004.09.007. PMID 15781190.
- Borges K, Sonnewald U (July 2012). “Triheptanoin–a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment?”. Epilepsy Res. 100 (3): 239–44. doi:10.1016/j.eplepsyres.2011.05.023. PMC 3422680. PMID 21855298.
External links
- “Triheptanoin”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT01379625 for “Study of Triheptanoin for Treatment of Long-Chain Fatty Acid Oxidation Disorder (Triheptanoin)” at ClinicalTrials.gov
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Dojolvi |
| Other names | UX007 |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| Drug class | Glycerolipids |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.009.681 |
| Chemical and physical data | |
| Formula | C24H44O6 |
| Molar mass | 428.610 g·mol−1 |
| 3D model (JSmol) | |
//////////Triheptanoin, Dojolvi, UX 007, FDA 2020, 2020 APPROVALS
Prescription Products














