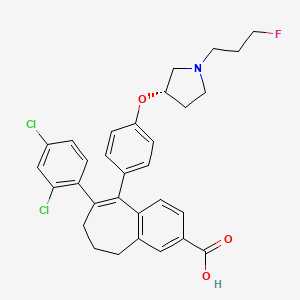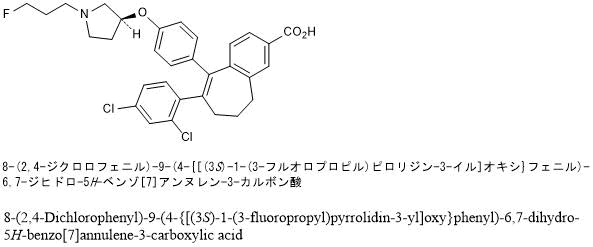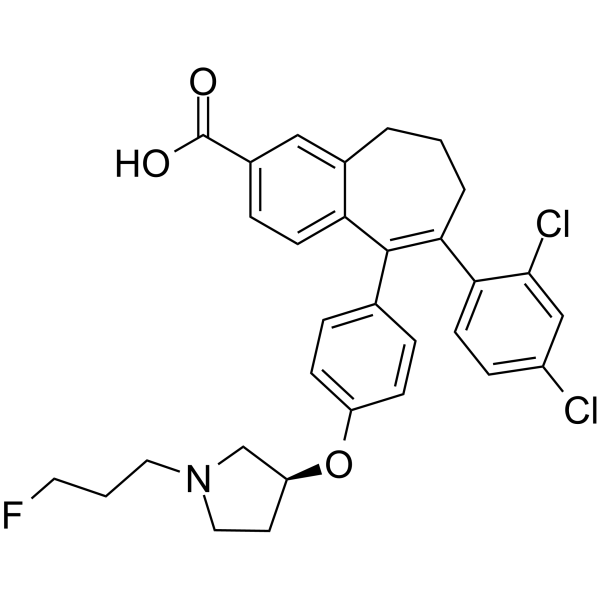
Amcenestrant (SAR 439859)
アムセネストラント
| Molecular Weight |
554.48 |
|---|---|
| Formula |
C31H30Cl2FNO3 |
| CAS No. |
6-(2,4-dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid
8-(2,4-dichlorophenyl)-9-(4-{[(3 S )-1-(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro – 5H- Benzo[7]annulene-3-carboxylic acid
8-(2,4-Dichlorophenyl)-9-(4-{[(3 S )-1-(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5 H -benzo [7]annulene-3-carboxylic acid
C31H30Cl2FNO3 : 554.48 [ 2114339-57-8 ] _ _ _ _ _ _
| Efficacy |
Antineoplastic, Selective estrogen receptor downregulator
|
|---|---|
| Comment |
Selective estrogen receptor downregulator (SERD)
Treatment of breast cancer |
SAR439859 (compound 43d) is an orally active, nonsteroidal and selective estrogen receptor degrader (SERD). SAR439859 is a potent ER antagonist and has ER degrading activity with an EC50 of 0.2 nM for ERα degradation. SAR439859 demonstrates robust antitumor efficacy and limited cross-resistance in ER+ breast cancer.
Amcenestrant is an orally available, nonsteroidal selective estrogen receptor degrader/downregulator (SERD), with potential antineoplastic activity. Upon oral administration, amcenestrant specifically targets and binds to the estrogen receptor (ER) and induces a conformational change that promotes ER degradation. This prevents ER-mediated signaling and inhibits both the growth and survival of ER-expressing cancer cells.
Amcenestrant is reported to be a selective estrogen receptor degrader (SERD) which has estrogen receptor antagonist properties and accelerates the proteasomal degradation of the estrogen receptor. Amcenestrant is under clinical investigation as an anticancer agent, in particular for treatment of breast cancer.
The compound and processes for preparation thereof are described in International Publication No. WO 2017/140669.
Crystalline forms are described in International Publication No. WO 2021/116074.
PAPER
Journal of Medicinal Chemistry (2020), 63(2), 512-52
https://pubs.acs.org/doi/10.1021/acs.jmedchem.9b01293
6-(2,4-Dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3- yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic Acid (43d).
To a solution of 6-(2,4-dichloro-phenyl)-5-[4-[1-(3-fluoropropyl)-pyrrolidin-3-yloxy]-phenyl]-8,9-dihydro-7H-benzocycloheptene-2-carboxylic acid methyl ester (42d) (80 mg, 140.72 μmol) in methanol (5 mL) was added 5 N NaOH (562.88 μL), the reaction mixture was heated to 60 °C for 5 h, and the solvent was removed under reduced pressure. The residue was taken up in water (10 mL), and aqueous HCl (5 M) was added to pH 7. The slurry was extracted with dichloromethane, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The solid was purified by column chromatography eluting with a mixture of dichloromethane, acetonitrile, and methanol (90/5/5 v/v/v) to give 60 mg (77%) of 6- (2,4-dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]- oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid (43d). 1 H NMR (400 MHz, DMSO-d6): 1.68 (m, 1H), 1.79 (dm, J = 25.3 Hz, 2 H), 2.07 to 2.23 (m, 5H), 2.38 (m, 1H), 2.46 (t, J = 7.2 Hz, 2H), 2.52 (m, 1H), 2.62 (m, 1H), 2.55 to 2.89 (m, 3H), 4.47 (td, J = 6.2 and 47.6 Hz, 2H), 4.72 (m, 1H), 6.63 (d, J = 8.9 Hz, 2H), 6.71 (m, 3H), 7.18 (d, J = 8.4 Hz, 1H), 8.26 (dd, J = 2.0 and 8.4 Hz, 1H), 7.58 (d, J = 2.0 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.79 (s, 1H), 12.3 (m, 1H). LCMS: 554 (M + H)+ .
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023287938&_cid=P22-LDCH1V-08798-1
Amcenestrant can be prepared according to methods known from the literature, for example U.S. Patent No. 9,714,221.
Example 1: Preparation of amorphous Amcenestrant
[00164] Amcenestrant (20 mg, prepared according to U.S. Patent No. 9,714,221) was dissolved in ethyl acetate (0.2 mL) at room temperature (25°C). Solution was left in opened flask at RT for 16 days, until all the solvent evaporated. Obtained solid was analyzed by XRPD.
PATENT
U.S. Patent No. 9,714,221
https://patents.google.com/patent/US9714221B1/en
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017140669
Example 51. 6-(2,4-dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid
Methode B:
Step 1 : 6-(2,4-dichloro-phenyl)-5-{4-[1-(3-fluoro-propyl)-pyrrolidin-3-yloxy]-phenyl}-8,9-dihydro-7H-benzocycloheptene-2-arboxylic acid methyl ester.
To a solution of methyl 8-bromo-9-(4-{[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate hydrobromide (D5) (150 mg, 298.56 μιηοΙ), in dioxane (12 ml) and water (2 ml), was added 2,4-dichlorophenyl-boronic acid (62.67 mg, 328.41 μηηοΙ), Cs2C03 (204.48 mg, 626.97 μηιοΙ), and Pd(dppf)CI2 (14.63 mg, 17.91 μιηοΙ). The reaction mixture was heated at 90°C for 3 hours, and partitioned between AcOEt and water. The phases were separated and the organic phase washed with brine, dried over MgS04 and concentrated under reduced pressure. The residue was purified by column chromatography eluting with a mixture of DCM, acetonitrile and MeOH (96/2/2; V/V/V) to give 80 mg (47%) of 6-(2,4-dichloro-phenyl)-5-{4-[1-(3-fluoro-propyl)-pyrrolidin-3-yloxy]-phenyl}-8,9-dihydro-7H-benzocycloheptene-2-arboxylic acid methyl ester.
LC/MS (m/z, MH+): 568
Step 2 : 6-(2,4-dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid
To a solution of 6-(2,4-dichloro-phenyl)-5-{4-[1-(3-fluoro-propyl)-pyrrolidin-3-yloxy]-phenyl}-8,9-dihydro-7H-benzocycloheptene-2-arboxylic acid methyl ester (80 mg, 140.72μιηο!) in MeOH (5 ml) was added a solution of NaOH (562.88 μΙ, 5 M) and the reaction mixture was heated at 60°C for 5 hours and the solvent removed under reduced pressure. The residue was taken up in water (10 ml) and aqueous HCI (5 M) added to pH
7. The slurry was extracted with DCM, dried over MgS04 and concentrated under reduced pressure. The solid was purified by column chromatography eluting with a mixture of DCM, acetonitrile and MeOH (90/5/5; V/V/V) to give 60 mg (77%) of 6-(2,4-dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid.
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019020559
Intermediate (c). Tert-butyl (3S)-3-[4-(4,4!5!5-tetramethyl-1 !3,2-dioxaborolan-2yl)phenoxy]pyrrolidine-1 -carboxylate
To a solution of commercially available 4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenol (a) (82.7 g, 364.51 mmol) in THF (2 L) was added under argon (R)-1 -N-Boc-3-hydroxypyrrolidine (b) (84.43 g, 437.41 mmol) followed by Ν,Ν,Ν’,Ν’-tetramethylazodicarboxamide (99.1 g, 546.77 mmol). The clear reaction mixture turned orange and triphenylphosphine (143.41 g, 546.77 mmol) was added. The reaction mixture was stirred at room temperature for 24 hours, meanwhile a precipitate of triphenylphosphine oxide formed (Ph3P=0). The reaction mixture was poured in water (1 .5 L) and extracted with ethyl acetate (AcOEt) (3×1 .5 L). Gathered organic phases were dried over magnesium sulfate (MgS04), filtered and concentrated under reduced pressure. The residue was taken up into diisopropylether (1 .5 L) and the solid formed (Ph3P=0) was filtered. The solvent was concentrated under reduced pressure and the residue purified by column chromatography eluting with a mixture of heptane with AcOEt (90/10; v/v) to give 145 g (100%) of tert-butyl (3S)-3-[4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy]pyrrolidine-1 -carboxylate (c) as a colorless oil.
1H NMR (400 MHz, DMSO-d6, δ ppm): 1 .27 (s : 12H); 1 .39 (s : 9H); 2.05 (m : 1 H); 2.14 (m : 1 H); 3.37 (3H); 3.55 (m : 1 H); 5.05 (s : 1 H); 6.94 (d, J = 8.4 Hz : 2H); 7.61 (d, J = 8.4 Hz : 2H)
Intermediate (d). (3S)-3-[4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2yl)phenoxy]pyrrolidine, hydrochloride
To a solution of (S)-tert-butyl 3-(4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy)pyrrolidine-1 -carboxylate (c) (80 g, 195.23 mmol) in MeOH (450 ml) was added slowly HCI 4N in dioxane (250 ml).
After 1 .5 hours, the reaction mixture was concentrated under reduced pressure and the residue was taken up into Et20 with stirring to give a solid which then was filtered and dried under vacuum to give 61.8 g (95%) of (3S)-3-[4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2yl)phenoxy]pyrrolidine, hydrochloride (d) as a white powder.
1H NMR (400 MHz, DMSO-d6, δ ppm): 1.28 (s : 12H); 2.10 (m : 1 H); 2.21 (m : 1 H); 3.31 (3H); 3.48 (m : 1 H); 5.19 (m : 1 H); 6.97 (d, J = 8.4 Hz : 2H); 7.63 (d, J = 8.4 Hz : 2H); 9.48 (s : 1 H); 9.71 (s : 1 H).
LC/MS (m/z, MH+): 290
Intermediate (e). (3S)-1 -(3-fluoropropyl)-3-[4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy]pyrrolidine
To a suspension of (S)-3-(4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy)pyrrolidine hydrochloride (d) (20 g, 61.42 mmol) in acetonitrile (100 ml), was added K2C03 (21 .22 g, 153.54 mmol) and 1 -iodo-3-fluoropropane (12.15 g, 61.42 mmol), under argon. The reaction
mixture was stirred at 40°C for 24 hours. After cooling to room temperature, the reaction mixture was filtered and washed with acetonitrile. The filtrate was concentrated under reduced pressure and the residue was taken up in DCM and the solid formed was filtered and washed with DCM. The filtrate was concentrated to give 21.5 g (100%) of (3S)-1 -(3-fluoropropyl)-3-[4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy]pyrrolidine (e) as a yellow foam.
1H NMR (400 MHz, DMSO-d6, δ ppm): 1.27 (s : 12H); 1 .77 (m : 2H); 1 .84 (m : 1 H); 2.27 (m : 1 H); 2.41 (m : 1 H); 2.49 (2H); 2.62 (dd, J = 2.6 and 10.4Hz : 1 H); 2.69 (m : 1 H); 2.83 (dd, J = 6.2 and 10.4Hz : 1 H); 4.47 (td, J = 6.2 and 47Hz : 2H) ; 4.99 (m : 1 H); 6.77 (d , J = 8.4 Hz : 2H); 7.58 (d, J = 8.4 Hz : 2H).
LC/MS (m/z, MH+): 350
Intermediate (B). 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl 2,2-dimethylpropanoate
To a solution of 2-hydroxy-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one (A) (1 .52 g, 8.63 mmol), in acetone (60 ml), was added K2C03 (1 .19 g, 8.63 mmol) and pivaloyl chloride (1.06 ml, 8.63 mmol). The reaction mixture was stirred at room temperature for 16 hours, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of heptane in AcOEt (100/0 to 85/15, v/v) to give 1.55 g (69%) of 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl 2,2-dimethylpropanoate (B) as a colorless oil.
1H NMR (400 MHz, DMSO-d6, δ ppm): 7.65 (d, 1 H); 7.10-7.04 (m, 2H); 2.95 (t, 2H); 2.68 (t, 2H); 1 .85-1 .65 (m, 4H).
LC/MS (m/z, MH+): 261
Intermediate (C). 9-(trifluoromethanesulfonyloxy)-6,7-dihydro-5H-benzo[7]annulen-3-yl 2,2-dimethylpropanoate
To a solution of 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl 2,2-dimethylpropanoate (B) (15 g, 57.62 mmol) in DCM (500 ml) was added dropwise under argon pyridine (7.28 ml, 86.43 mmol) and trifluoromethanesulfonic anhydride (19.58 ml, 1 15.24 mmol). The reaction mixture was stirred at room temperature for 2 hours and ice (200 g) was added. The phases were separated, the aqueous phase was washed with DCM and the gathered organic phases were dried over MgS04, filtered and evaporated under reduced pressure to give 22 g (97%) of 9-(trifluoromethanesulfonyloxy)-6,7-dihydro-5H-benzo[7]annulen-3-yl 2,2-dimethylpropanoate (C) as a white solid.
LC/MS (m/z, MH-): 391
Intermediate (D). 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-yl-2,2-dimeth lpropanoate
To a solution of 9-(trifluoromethanesulfonyloxy)-6,7-dihydro-5H-benzo[7]annulen-3-yl-2,2-dimethylpropanoate (C) (22 g, 56.07 mmol) and (3S)-1 -(3-fluoropropyl)-3-[4-(tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy]pyrrolidine (e) (20.56 g, 58.87 mmol) in dioxane (420 ml) and water (120 ml) was added under argon Pd(dppf)CI2 (2.75 g, 3.36 mmol) and Cs2C03 (36.57 g, 1 12.13 mmol). The reaction mixture was stirred for 1 hour at room temperature and was partitioned between water and DCM. The aqueous phase was washed with DCM and the gathered organic phases dried over MgS04, filtered and concentrated under reduced pressure. The residue was purified by column chromatography eluting with a gradient of MeOH in DCM (0 to 5%; V/V) to give 31 g (100 %) of 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-yl-2,2-dimethylpropanoate (D).
LC/MS (m/z, MH+): 466
Intermediate (E). 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-ol
To a solution under argon of 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-yl-2,2-dimethylpropanoate (D) (24.8 g, 53.26 mmol) in MeOH (300 ml), was added NaOH 5M (23 ml, 1 15.00 mmol). The reaction mixture was stirred for 2 hours at room temperature. pH was then adjusted to 7 by addition of 6N aqueous HCI solution. The MeOH was concentrated under reduced pressure, then DCM was added. The organic phase was dried over MgS04, and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of DCM/ MeOH from 100/0 to 95/05 to give 18.8 g (93%) of 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-ol (E) as a beige solid.
LC/MS (m/z, MH+): 382
Intermediate (F). 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-yl trifluoromethanesulfonate
To a solution of 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-ol (E) (20.6 g, 54.00 mmol) in DCM (200 ml) and pyridine (6.55 ml, 81 .00 mmol), cooled to 5°C (ice bath), was added dropwise trifluoromethanesulfonic anhydride (18.93 ml, 108.00 mmol) under argon, and the reaction temperature was maintained <15°C. The ice bath was removed, and the brown suspension was stirred at room temperature for 2 hours. Ice (200 g) and DCM (200 ml) were added and the phases separated. The organic phase was dried over MgS04, and concentrated under reduced pressure. The residue was
purified by flash chromatography eluting with a gradient of DCM/MeOH from 100/0 to 95/05 to give 24.7 g (89.1 %) of 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-yl trifluoromethanesulfonate (F) as a brown oil.
LC/MS (m/z, MH+): 514
Intermediate (G). Methyl 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate
To a solution of 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulen-3-yl trifluoromethanesulfonate (F) (10.1 g, 19.67 mmol) in DMF (66 ml) and MeOH (33 ml), were added Pd(dppf)CI2 (909 mg, 1.18 mmol) and diisopropylethylamine (7.21 ml). The black suspension was carbonylated in an autoclave at 70°C under 5 bars of CO for 5 hours. The reaction mixture was filtered, then the filtrate was partially concentrated under reduced pressure. The residue was partitioned between AcOEt and water. The organic phase was washed with water (2x 100 ml), dried over MgS04, and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of DCIW MeOH from 100/0 to 95/05 to give 7.13 g (86%) of methyl 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate (G) as a brown gum.
LC/MS (m/z, MH+): 424
Intermediate (A1 ). 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yltrifluoromethanesulfonate
To a solution of commercially available 2-hydroxy-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one (A) (18.5 g, 105 mmol) in DCM (185 ml) and lutidine (13.35 ml, 1 13.505 mmol), cooled at 5°C under argon, was added dropwise trifluoromethanesulfonic anhydride (20.22 ml,
123.29 mmol) while keeping temperature between 10 and 20°C. The reaction mixture was stirred for 1 hour at 5°C then at room temperature for 1 hour.
Then, ice (200 g) was added and the slurry partitioned between water and DCM. The organic phase was washed with aqueous NaHC03 solution, dried over MgS04, filtered off and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of heptane/AcOEt from 100 to 90/10 to give 28.2 g (87%) of 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl trifluoromethanesulfonate (A1 ) as an orange oil. LC/MS (m/z, MH+): 309
Intermediate (B1 ). Methyl 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-carboxylate
To a solution of 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl trifluoromethanesulfonate (A1 ) (5.03 g, 16.32 mmol) in DMF (24 ml) and MeOH (12 ml), were added Pd(dppf)CI2 (754 mg, 0.98 mmol) and diisopropylethylamine (6 ml). The black suspension was carbonylated in an autoclave at 70°C under 5 bars of CO for 2.5 hours. The reaction mixture was filtered, then the filtrate was partially concentrated under reduced pressure, and the residue, was partitioned between AcOEt and water. The organic phase was washed with water (2x 75 ml) and aqueous HCI 0.5 N, dried over MgS04 and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of heptane/AcOEt from 100/0 to 90/10 to give 3.4 g (95%) of methyl 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-carboxylate (B1 ) as a colorless oil.
LC/MS (m/z, MH+): 219
Intermediate (C1 ). Methyl 9-(trifluoromethanesulfonyloxy)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate
To a solution of methyl 5-oxo-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-carboxylate (B1 ) (18,19 g, 83,34 mmol) in DCM (500 ml) and anhydrous pyridine (1 1 ml, 130,56 mmol), cooled at 5°C under argon, was added dropwise trifluoromethanesulfonic anhydride (30 ml, 176,54 mmol). The reaction mixture, a thick suspension, was stirred at room temperature for 24 hours, then ice was added and partitioned between water and DCM. The organic phase was dried over MgS04, filtered off and concentrated under reduced pressure to give 29 g (100%) of methyl 9-(trifluoromethanesulfonyloxy)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate (C1 ) as a yellow gum.
LC/MS (m/z, MH+): 351
Intermediate (G). Methyl 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate
To a solution of methyl 9-(trifluoromethanesulfonyloxy)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate (C1 ) (29 g, 82.9 mmol), (3S)-1 -(3-fluoropropyl)-3-[4-(tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenoxy]pyrrolidine (e) (28.9 g, 82.9 mmol), in dioxane (225 ml) were added Pd(dppf)CI2 under argon, complex with DCM (3.73 g, 4.57 mmol) and Cs2C03 1 .5 M aqueous solution (1 1 1.12 ml, 166.68 mmol). The reaction mixture was stirred at 60°C for 1 hour.
After cooling to room temperature, the reaction mixture was poured into a mixture of water (500 ml) and AcOEt (400ml). The organic phase was washed with brine, dried over MgS04, filtered on celite and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of DCM/MeOH from 100/0 to 95/05 to give 23 g (65%) of methyl 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate (G) as a brown gum.
LC/MS (m/z, MH+): 424
Intermediate (H). Methyl 8-bromo-9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro-5H-benzo[7]annulene-3-carboxylate hydrobromide
To a solution of methyl 9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro- 5H-benzo[7]annulene-3-carboxylate (G) (13.93 g, 32.89 mmol), in DCM (150 ml) was added under argon pyridinium tribromide (15.78 g, 44.41 mmol). The reaction mixture was stirred for 1 hour at room temperature. Water (200 ml) was added, organic phase was then dried over MgS04, and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with a gradient of DCM/MeOH from 100/0 to 95/05 to give 16.4 g (85%) of methyl 8-bromo-9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro- 5H-benzo[7]annulene-3-carboxylate hydrobromide (H) as a yellow meringue.
LC/MS (m/z, MH+): 502
Intermediate (I). 6-(2,4-dichloro-phenyl)-5-{4-[1 -(3-fluoro-propyl)-pyrrolidin-3-yloxy]-phenyl}- -dihydro-7H-benzocycloheptene-2-arboxylic acid methyl ester.
To a solution of methyl 8-bromo-9-(4-{[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7- dihydro-5H-benzo[7]annulene-3-carboxylate hydrobromide (H) (150 mg, 298.56 μηηοΙ), in dioxane (12 ml) and water (2 ml), was added 2,4-dichlorophenyl-boronic acid (62.67 mg, 328.41 μηιοΙ), Cs2C03 (204.48 mg, 626.97 μπιοΙ), and Pd(dppf)CI2 (14.63 mg, 17.91 mol). The reaction mixture was heated at 90°C for 3 hours, and partitioned between AcOEt and water. The phases were separated and the organic phase washed with brine, dried over MgS04 and concentrated under reduced pressure. The residue was purified by column
chromatography eluting with a mixture of DCM, acetonitrile and MeOH (96/2/2; V/V/V) to give 80 mg (47%) of 6-(2,4-dichloro-phenyl)-5-{4-[1 -(3-fluoro-propyl)-pyrrolidin-3-yloxy]-phenyl}-8,9-dihydro-7H-benzocycloheptene-2-arboxylic acid methyl ester (I).
LC/MS (m/z, MH+): 568
Compound (1 ). 6-(2,4-dichlorophenyl)-5-[4-[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulen -2-carboxylic acid
To a solution of 6-(2,4-dichloro-phenyl)-5-{4-[1 -(3-fluoro-propyl)-pyrrolidin-3-yloxy]-phenyl}-8,9-dihydro-7H-benzocycloheptene-2-arboxylic acid methyl ester (I) (80 mg, 140.72 μηηοΙ) in MeOH (5 ml) was added a solution of NaOH (562.88 μΙ, 5 M) and the reaction mixture was heated at 60°C for 5 hours and the solvent removed under reduced pressure. The residue was taken up in water (10 ml) and aqueous HCI (5 M) added to pH 7. The slurry was extracted with DCM, dried over MgS04 and concentrated under reduced pressure. The solid was purified by column chromatography eluting with a mixture of DCM, acetonitrile and MeOH (90/5/5; V/V/V) to give 60 mg (77%) of 6-(2,4-dichlorophenyl)-5-[4-[(3S)-1 -(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid. 1H NMR (400 MHz, DMSO-d6, δ ppm): 1 .68 (m, 1 H); 1 ,79 (dm, J=25.3 Hz, 2 H); 2.07 to 2.23 (m, 5 H); 2.38 (m, 1 H); 2.46 (t, J=7.2 Hz, 2 H); 2.52 (m, 1 H); 2.62 (m, 1 H); 2.55 to 2.89 (m, 3 H); 4.47 (td, J=6.2 and 47.6 Hz, 2 H); 4.72 (m, 1 H); 6.63 (d, J=8.9 Hz, 2 H); 6.71 (m, 3 H); 7.18 (d, J=8.4 Hz, 1 H); 8.26 (dd, J=2.0 and 8.4 Hz, 1 H); 7.58 (d, J=2,0 Hz, 1 H); 7.63 (d, J=8.4 Hz, 1 H); 7.79 (s, 1 H); 12.3 (m, 1 H)
LC/MS (m/z, MH+): 554
//////////////
Admin note for myself . I am up for Grabs
I myself Dr Anthony Melvin Crasto Looking for a post retirement assignment as Advisor API & INT, Chem.
With 36 yrs rich experience, about dozen patents, 10000plus steps covered, 200 API targets, 30 plus products commercialization in plant in full career. Hands on knowledge of Synthesis, Process, scaleup, cost reduction, DOE , softwares etc
Kindly contact me
Dr Anthony Melvin Crasto
+919321316780
amcrasto@gmail.com
About myself
Dr Anthony Crasto
click on my website to know about me
Read http://amcrasto.weebly.com/
Also http://amcrasto.weebly.com/awards.html
Also
http://amcrasto.weebly.com/felicitations.html
1000 lakh google hits, 100lakh blog views, 10 lakh viewers in USA alone, all in 7 continents, 226 countries, 30 Indian and International awards, helping millions across the world