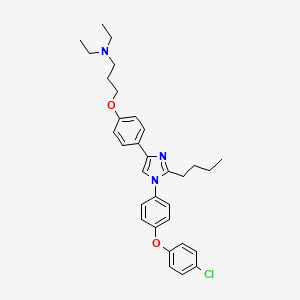
Azeliragon
C32H38ClN3O2, 532.1 g/mol
CAS 603148-36-3
TTP488
UNII-LPU25F15UQ
LPU25F15UQ
TTP-488; PF-04494700
3-[4-[2-butyl-1-[4-(4-chlorophenoxy)phenyl]imidazol-4-yl]phenoxy]-N,N-diethylpropan-1-amine
MOA:RAGE inhibitor
Indication:Alzheimer’s disease (AD)
Status:Phase III (Active), Dementia, Alzheimer’s type
Company:vTv Therapeutics (Originator)
Azeliragon
Azeliragon is in phase III clinical for the treatment of Alzheimer’s type dementia.
Azeliragon was originally by TransTech Pharma (now vTv Therapeutics), then licensed to Pfizer in 2006.
Pfizer discontinued the research in 2011, now vTv Therapeutics continues the further reaserch.
vTv Therapeutics (previously TransTech Pharma) is developing azeliragon, an orally active antagonist of the receptor for advanced glycation end products (RAGE), for the treatment of Alzheimer’s disease (AD) in patients with diabetes. In June 2019, this was still the case .
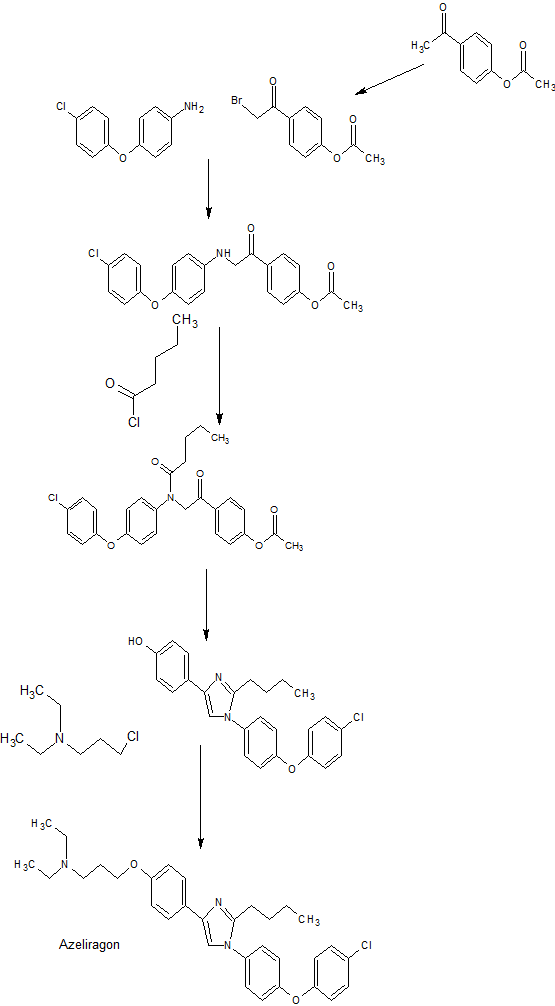
Azeliragon was originally developed at TransTech Pharma. In September 2006, Pfizer entered into a license agreement with the company for the development and commercialization of small- and large-molecule compounds under development at TransTech. Pursuant to the collaboration, Pfizer gained exclusive worldwide rights to develop and commercialize TransTech’s portfolio of RAGE modulators, including azeliragon.
1. WO03075921A2.
2. US2008249316A1.
US 20080249316

Azeliragon (TTP488) is an orally bioavailable small molecule that inhibits the receptor for advanced glycation endproducts (RAGE). A Phase 2 clinical trial to evaluate azeliragon as a potential treatment of mild-AD in patients with type 2 diabetes is ongoing. The randomized, double-blind, placebo-controlled multicenter trial is designed as sequential phase 2 and phase 3 studies operationally conducted under one protocol. For additional information on the study, refer to NCT03980730 at Clinicaltrials.gov.
RAGE is an immunoglobulin-like cell surface receptor that is overexpressed in brain tissues of patients with AD. The multiligand nature of RAGE is highlighted by its ability to bind diverse ligands such as advanced glycation end-products (AGEs), linked to diabetic complications and β-amyloid fibrils, a hallmark of AD. The association between type 2 diabetes and AD is well documented. A linear correlation between circulating hemoglobin A1c (HbA1c) levels and cognitive decline has been demonstrated in the English Longitudinal Study of Ageing.
PATENT
WO-2019190823
Novel crystalline forms of [3-(4-{2-butyl-1-[4-(4-chlorophenoxy)phenyl]-1H-imidazol-4-yl}phenoxy)-propyl]-diethylamine and its salt ( azeliragon ) (deignated as forms III and IV) as RAGE inhibitors useful for treating psoriasis, rheumatoid arthritis and Alzheimer’s disease.
The Receptor for Advanced Glycation Endproducts (RAGE) is a member of the immunoglobulin super family of cell surface molecules. Activation of RAGE in different tissues and organs leads to a number of pathophysiological consequences. RAGE has been implicated in a variety of conditions including: acute and chronic inflammation (Hofmann et al., Cell 97:889-901 (1999)), the development of diabetic late complications such as increased vascular permeability (Wautier et al., J. Clin. Invest. 97:238-243 (1995)), nephropathy (Teillet et al., J. Am. Soc. Nephrol. 11 : 1488- 1497 (2000)), atherosclerosis (Vlassara et. al., The Finnish Medical Society DUODECIM, Ann. Med. 28:419-426 (1996)), and retinopathy (Hammes et al., Diabetologia 42:603-607 (1999)). RAGE has also been implicated in Alzheimer’s disease (Yan et al., Nature 382: 685-691 , (1996)), erectile dysfunction, and in tumor invasion and metastasis (Taguchi et al., Nature 405: 354-357, (2000)).
Binding of ligands such as advanced glycation endproducts (AGEs), S100/calgranulin/EN-RAGE, b-amyloid, CML (Ne-Carboxymethyl lysine), and amphoterin to RAGE has been shown to modify expression of a variety of genes. For example, in many cell types interaction between RAGE and its ligands generates oxidative stress, which thereby results in activation of the free radical sensitive transcription factor NF-kB, and the activation of NF-kB regulated genes, such as the cytokines IL- 1 b, TNF- a, and the like. In addition, several other regulatory pathways, such as those involving p21 ras.
MAP kinases, ERK1 and ERK2, have been shown to be activated by binding of AGEs and other ligands to RAGE. In fact, transcription of RAGE itself is regulated at least in part by NF-kB. Thus, an ascending, and often detrimental, spiral is fueled by a positive feedback loop initiated by ligand binding. Antagonizing binding of physiological ligands to RAGE, therefore, is our target, for down-regulation of the pathophysiological changes brought about by excessive concentrations of AGEs and other ligands for RAGE.
Pharmaceutically acceptable salts of a given compound may differ from each other with respect to one or more physical properties, such as solubility and dissociation, true density, melting point, crystal shape, compaction behavior, flow properties, and/or solid state stability. These differences affect practical parameters such as storage stability, compressibility and density (important in formulation and product manufacturing), and dissolution rates (an important factor in determining bio-availability). Although U.S. Patent No. 7,884,219 discloses Form I and Form II of COMPOUND I as a free base, there is a need for additional drug forms that are useful for inhibiting RAGE activity in vitro and in vivo, and have properties suitable for large-scale manufacturing and formulation. Provided herein
PATENT
WO03075921
PATENT
WO2019190822
PATENT
WO2008123914
Publications
Links to the following publications and presentations, which are located on outside websites, are provided for informational purposes only and do not constitute the opinions or views of vTv Therapeutics
- Burstein, A. H., Brantley, S. J., Dunn, I. , Altstiel, L. D. and Schmith, V. (2019), Assessment of Azeliragon QTc Liability Through Integrated, Model‐Based Concentration QTc Analysis. Clinical Pharmacology in Drug Development
- Burstein AH, Sabbagh M, Andrews R, Valcarce C, Dunn I, Altstiel L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J Prev Alz Dis 2018;5(2):149-154
- Sabbagh MN, Agro A, Bell J, Aisen PS, Schweizer E, Galasko D. PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzeimer disease. Alzheimer Dis Assoc Disord 2011;25(3):206-212
- Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS; Alzheimer’s Disease Cooperative Study. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology 2014; 82(17):1536-42
- Burstein AH, Grimes I, Galasko DR, Aisen PS, Sabbagh M, Mjalli AM. Effect of TTP488 in patients with mild to moderate Alzheimer disease. BMC Neurol 2014;14:12
- Chen Y., Akirav E, Chen W, Henegariu O, Moser B, Desai D, Shen J, Webster J, Andrews R, Mjalli A, Rothlein R, Schmidt AM, Clynes R, Herold K. RAGE Ligation Affects T Cell Activation and Controls T Cell Differentiation. J Immunol 2008; 181:4272-4278
Presentations and Posters
Links to the following publications and presentations, which are located on outside websites, are provided for informational purposes only and do not constitute the opinions or views of vTv Therapeutics
- Valcarce C, Dunn I, Burstein A. Linking Diabetes and Alzheimer’s Disease through RAGE. A Retrospective Analysis of Azeliragon Phase 2 and Phase 3 Studies. Presented at the 2019 Alzheimer’s Association International Conference (AAIC). July 17, 2019. Los Angeles, California
- Valcarce C, Dunn I, Soeder T, Burstein A. Inflammatory biomarkers, brain volumetric MRI, FDG-Pet results in patients with type 2 diabetes in azeliragon phase 3 trial in mild Alzheimer’s disease (AD). Presented at 14th International Conference on Alzheimer’s & Parkinson’s Diseases. March 30, 2019. Lisbon, Portugal
- Sabbagh M, Dunn I, Gooch A, Soeder T, Kieburtz K, Valcarce C, Altstiel L, Burstein A. Safety and efficacy results from the phase 3, multicenter, 18-month STEADFAST trial of azeliragon in participants with mild Alzheimer’s disease. Presented at 2018 CTAD. October 26, 2018. Barcelona, Spain
- Valcarce C, Dunn I, Soeder T, Burstein A. Is RAGE the missing link between diabetes and dementia? Results from a subgroup analysis of the STEADFAST trial. Presented at 2018 CTAD. October 24, 2018. Barcelona, Spain
- Burstein A, Dunn I, Altstiel L. Analysis of treatment emergent adverse event incidences in phase 2 study of azeliragon reveal potential attenuation of psychiatric system organ class (SOC) adverse events and expected drug effects in gastrointestinal SOC. Presented at 2017 CTAD. November 2, 2017. Boston, MA
- Burstein A, Lamson M, Sale M, Brantley S, Gooch A, Dunn I, Altstiel L. Effect of CYP2C8 and CYP3A4 inhibition and CYP induction on the pharmacokinetics of azeliragon. Presented at 2017 CTAD. November 2, 2017. Boston, MA
- Burstein A, Gooch A, Brantley S, Lamson M, Dunn I, Altstiel L. Effect of mild or moderate hepatic impairment on the clearance of azeliragon. Presented at 2017 CTAD. November 2, 2017. Boston, MA
- Burstein A, Dunn I, Soeder T, Sabbagh M, Altstiel L. Azeliragon Phase 2b Survival Analysis Supports Beneficial Effects on Delaying Time to Cognitive Deterioration in Patients with Mild Alzheimer’s Disease. Presented at 2016 Alzheimer’s Association International Conference (AAIC). July 27, 2016. Toronto, Canada
- Sabbagh MN, Burstein A, Dunn I, Valcarce C, Rose EA, Doody R, Sano M, Schneider LS, Galasko DR. “TTP488: From futile to fast track” AAIC 2015
- Burstein A, Galasko D, Aisen P, Thomas R, Grimes I, Clark DJ, Mjalli A, Orlande C. Evaluation of the relationship between TTP488 plasma concentrations and changes in ADAS-cog relative to placebo. Alzheimer’s & Dementia 2013;9(4, Supplement): 279-280
- Kostura MJ, Kindy MS, Burstein A, Valcarce C, Polisetti D, Andrews R, Mjalli AM. Efficacy of RAGE antagonists in murine model of Alzheimer’s disease. Alzheimer’s & Dementia 2014;10(4, Supplement): 638-639.
///////////Azeliragon, psoriasis, rheumatoid arthritis, Alzheimer’s disease, TTP-488, PF-04494700, RAGE inhibitors, TransTech Pharma, PHASE 3, Dementia, Alzheimer’s type,
CCCCC1=NC(=CN1C2=CC=C(C=C2)OC3=CC=C(C=C3)Cl)C4=CC=C(C=C4)OCCCN(CC)CC