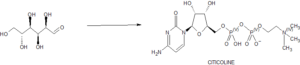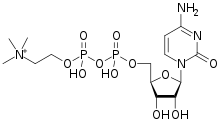
CITICOLINE
- Molecular FormulaC14H26N4O11P2
- Average mass488.324 Da
Citicoline (INN), also known as cytidine diphosphate-choline (CDP-Choline) or cytidine 5′-diphosphocholine is an intermediate in the generation of phosphatidylcholine from choline, a common biochemical process in cell membranes. Citicoline is naturally occurring in the cells of human and animal tissue, in particular the organs.
Studies suggest that CDP-choline supplements increase dopamine receptor densities.[1] Intracerebroventricular administration of citicoline has also been shown to elevate ACTH independently from CRH levels and to amplify the release of other HPA axis hormones such as LH, FSH, GH and TSH in response to hypothalamic releasing factors.[2] These effects on HPA hormone levels may be beneficial for some individuals but may have undesirable effects in those with medical conditions featuring ACTH or cortisol hypersecretion including PCOS, type II diabetes and major depressive disorder.[3][4]
Citicoline was originally developed in Japan for stroke. Citicoline or its sodium salt was later introduced as a prescription drug in many European countries. In these countries it is now frequently prescribed for thinking problems related to circulation problems in the brain. In the US, citicoline is marketed as a dietary supplement. Citicoline or its sodium salt is used for Alzheimers disease and other types of dementia, head trauma, cerebrovascular disease such as stroke, age-related memory loss, Parkinsons disease, and glaucoma.
Citicoline sodium is chemically known as 5-0-[hydroxy({hydroxy[2-(trimethylammonio)ethoxy]phosphoryl}oxy)phosphoryl]cytidine sodium which is represented by formula I,
There are many process described in the art for the preparation of citicoline. Japanese patent 51028636 describes a process for the preparation of citicoline by neutralisation of Calcium salt of phosphorylcholine chloride with 98% H2SO4 to make phosphorylcholine chloride, which is further treated with cytidine-5-phosphate in presence of DCC and pyridine at 70 C to obtain citicoline hydrate. The drawback of this process is that citicoline is very unstable
in this harsh reaction condition such as formamide, 98% H2SO4 and high temperature of 70 C.
Chinese patent 1944661 describes an enzymatic process for the preparation of citicoline which involves twice pH adjustment to precipitatethe product,filtration of the solids, charcolisation, washing with pure water, eluting through chloride type ion exchange resin with water ethanol/alcohol reagents, desalting the eluate, decoloring and collecting the liquid, vacuum-concentration of the eluate by adding an alcohol solvent to get the solid to obtain the crude product and dissolving the crude product, microfiltering, ultrafiltering, adding an alcoholic solvent, to obtain the wet productand drying to obtain the final product. The primary disadvantage of this process is that the above reaction involves water and ethanol mixture for elution of ion exchange column and also vacuum concentration of water ethanol mixture which requires high energy, more time, leads to decomposition of product and also leads to the formation of more effluent hence it is not suitable for large scale production.
The primary disadvantage of this process is that the above reaction involves water and ethanol mixture for elution of ion exchange column and also vacuum concentration of water ethanol mixture which requires high energy, more time, leads to decomposition of product and also leads to the formation of more effluent hence it is not suitable for large scale production.
US20090286284 describes a microbial process for preparation of citicoline. This patent also discloses a process for purification of citicoline by passing through acidic cation exchange and anion exchange resin. The drawback of this process is that in this process citicoline is passed through cation /anion exchange resin in free form which is unstable and liable to formation of unwanted impurities. Therefore for the purification it needs very high volume of resin (200 times) and high volume (100 times) of solvent. This process further needs reconcentration of huge volume of solvents, which is time taking and energy consuming.
Chemical and Pharmaceutical Bulletin 1971, 19(5), 1011-16 describes a process for the preparation of citicoline by direct condensation of cytidine 5-
monophosphate and choline phosphate by using p-toluenesulfonyl chloride or methanesulfonyl chloride combined with DMF. After completion of reaction the mass was diluted with water, pH was adjusted with ammonia solution to 9.5 and product was purified by using Dowex-1 ion exchange resin by eluting with formic acid. Another Chemical and Pharmaceutical Bulletin 1971, 19(12), 2499-71 describes a process for the preparation of citicoline by direct condensation cytidine 5-monophosphate and choline phosphate in presence of thionyl chloride and DMF.The product obtained was further purified by using Dowex-1 ion exchange resin by eluting with formic acid.
Journal of Biological Chemistry, 1956, 185-191 describes a process for the preparation of citicoline by direct condensation5-cytidylic acid and phosphorylcholine in a mixture of water and pyridine in presence of DCC, stirred for few days by adding DCC in lots, after completion of reaction, reaction mass was diluted with water and filtered. The pH of the filtrate was adjusted 8-9 using 0.5N KOH, diluted further with water and passed through Dowex-1 formate resin by eluting with formic acid and water.
The drawbacks of these processes are that they use hazardous reagents such as p-toluenesulfonyl chloride, methanesulfonyl chloride, thionyl chloride etc. Hence they are not suitable for large scale production. Also, the prior art processes pass citicoline solution, without isolating it, to ion exchange resins for purification. During this process most of the inorganic impurities present along with citicoline or its salt pass through the column, thus making purification difficult.
SYN
Journal of Chemical Research, 40(6), 358-360; 2016
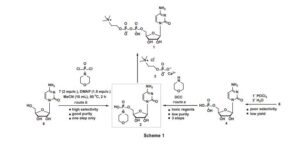
An improved, three-step synthesis of cytidine diphosphate choline (CDP-choline) from cytidine was achieved in 68% overall yield. Selective phosphorylation of cytidine was accomplished by the use of morpholinophosphodichloridate to give cytidine-5′-phosphomorpholide, which was condensed with choline phosphate chloride in the presence of a catalytic amount of H2SO4 to give CDP-choline. The intermediates and products could be efficiently purified by recrystallisation, thus avoiding the use of chromatography at all stages. The reaction could be scaled up to 200 g in 64% overall yield, making this route attractive for industrial application.

Cytidine diphosphate choline (CDP-choline 1) is a nucleotide coenzyme and serves as a choline donor in the biosynthesis of lipids,1 lecithins,2 and sphingomyelin.3 It is a clinical drug for the treatment of several illnesses involving disturbance of the central nervous system, in particular, for regaining a patient’s consciousness and for treatment of neuropsychic symptoms occurring during skull traumas and brain surgery.4 Among various methods for the synthesis of CDP-choline in the literature, the current preferred method is via the condensation of cytidine-5’-phosphomorpholide (2) with choline phosphate chloride (3) under mild reaction conditions.5–7 Compound 2 was synthesised from 5’-cytidine monophosphate (4) and morpholine in the presence of DCC (N,N’-dicyclohexylcarbodiimide)8 or via the controlled hydrolysis of cytidine-5’-phosphodichloride (5) followed by P–N bond formation with morpholine (Scheme 1, route a).7 However, DCC is toxic and converted into urea which is difficult to separate from the mixture, thus leading to poor purity of product. Furthermore, phosphorylation with POCl3 always meets with side reactions from the 2’ or 3’ hydroxyls and detracts from the acceptance of this method in industry.9 In the context of ongoing projects on the synthesis of nucleoside drugs,10–14 herein we report the synthesis of CDP-choline via the selective phosphorylation of 6 wiResults and discussion Central to our approach for the synthesis of CDP-choline is the selective phosphorylation of 6 using sterically-hindered 7 as phosphorylation regent. 7 was synthesised by the direct phosphorylation of morpholine with POCl3 , a compound whose utility for the conversion of alcohols and amines into various phosphorylation derivatives.15 Due to the reactivity of three chloro atoms in POCl3 , gradually adding POCl3 to excess morpholine avoids the bifunctional reaction exclusively. After reaction, 7 could be purified by fractional distillation to yield as a colourless oil (b.p. 124–126 °C at 1.33 KPa). Due to the presence of the electron-donating morpholino group, 7 displays lower reactivity than POCl3 and could tolerate moisture and air better. Usefully, 7 could be synthesised on the 200 g scale and stored at 4 °C. The major concern of utilising 7 as phosphorylation reagent is its selectivity for the 5’ hydroxyl group. We therefore assessed the selectivity for 5’ hydroxylation using 6 and 7 in the presence of various organic bases. After phosphorylation, H2 O was added to destroy the excess of 7, and 2 was obtained in a single step. The solvent, the base, temperature and the ratio of substrates were evaluated and the results are summarised in Table 1
https://journals.sagepub.com/doi/pdf/10.3184/174751916X14628025243831
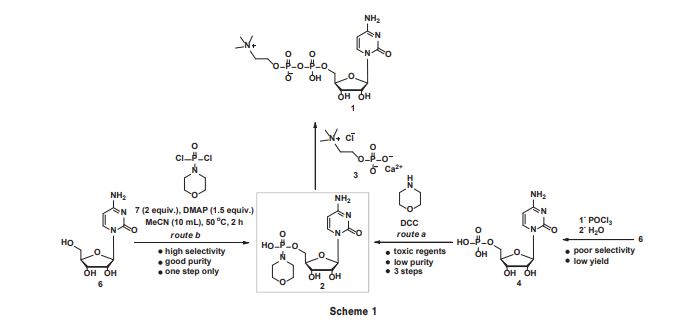
Cytidine-5’-phosphomorpholide (2): Cytidine (0.243 g, 1.0 mmol) and DMAP (0.183 g, 1.5 mmol) in MeCN (10 mL) were stirred slowly and cooled to 0 °C, and 7 (2.0 mmol) was added slowly. The mixture was heated to 50 °C and kept at this temperature for 2 h. The solvent was removed in vacuo and the residue was purified by recrystallisation from EtOH to give 2 as a white semi-solid (0.318 g); yield 81%; m.p. 62–64 °C; 1 H NMR (400 MHz, DMSO-d6 ) δ 8.43 (d, J = 7.6 Hz, 1H), 7.39 (s, 2H), 7.19 (d, J = 7.6 Hz, 1H), 5.77 (d, J = 2.8 Hz, 1H), 5.51 (d, J = 4.8 Hz, 1H), 5.18 (t, J = 5.2 Hz, 1H), 5.08 (d, J = 5.6 Hz, 1H), 3.76–3.71 (m, 1H), 3.61–3.56 (m, 1H), 3.45–3.42 (m, 4H), 3.03–2.99 (m, 4H); 13C NMR (100 MHz, DMSO-d6 ) δ 166.5, 157.8, 145.9, 141.6, 88.1, 86.2, 74.3, 70.7, 65.1, 65.0, 60.3, 60.2; HRMS calcd for C13H22N4 O8 P [M + H]+ 393.1170, found: 393.1172.
CDP-choline (1): 2 (0.392 g, 1.0 mmol) was added to MeOH (10 mL) followed by the addition of 3 (0.310 g, 1.2 mmol) and was stirred at room temperature for 10 min. Then 98% H2 SO4 (0.005 mL, 10 mol%) was added. The mixture was kept at 50 °C for 3 h. The solvent was removed in vacuo and the residue was purified by recrystallisation from EtOH to give 1 as a white solid (0.410 g); yield 85%. 1 H NMR (400 MHz, D2 O) δ 7.86 (s, 2H), 6.04 (d, J = 5.2 Hz, 1H), 5.91 (d, J = 5.2 Hz, 1H), 4.32 (brs, 2H), 4.26–4.22 (m, 2H), 4.18 (brs, 2H), 4.11 (t, J = 3.2 Hz, 1H), 3.60 (t, J = 2.4 Hz, 2H), 3.14 (s, 9H); 13C NMR (100 MHz, D2 O) δ 166.1, 157.7, 141.5, 96.6, 89.3, 82.6, 74.1, 69.3, 66.0, 65.9, 64.8, 59.9, 54.0; HRMS calcd for C14H27N4 O11P2 [M + H]+ 489.1146, found: 489.1140.
| 1H NMR | (400 MHz. D2O) δ 7.86 (s, 2H). 6.04 (d. J = 5.2 Hz, 111). 5.91 (d. J = 5.2 Hz. 1Hj, 4.32 (brs. 2H), 4.26-4.22 (m, 2H). 4.18 (brs, 2H), 4.11 (t. J = 3.2 Hz. 1H). 3.60 (t. J = 2.4 Hz. 2H), 3.14 (s, 9H) |
| 13C NMR | (100 MHz. D2O) δ 166.1. 157.7, 141.5,96.6.89.3,82.6,74.1.69.3,66.0, 65.9, 64.8. 59~9. 54.0 |
| HRMS | calcd for C14H27N4O11P2 (M + H]+ 489.1146. found: 489.1140 |
| State | white solid |
SYN
SYN
Indian Pat. Appl., 2014MU00923

SYN
CN 111647636

Syn
Biotechnology and Bioengineering, 117(5), 1426-1435; 2020
https://onlinelibrary.wiley.com/doi/10.1002/bit.27291
Cytidine-5′-diphosphocholine (CDP-choline) is a widely used neuroprotective drug for multiple indications. In industry, CDP-choline is synthesized by a two-step cell culture/permeabilized cell biotransformation method because substrates often do not enter cells in an efficient manner. This study develops a novel one-step living cell fermentation method for CDP-choline production. For this purpose, the feasibility of Pichia pastoris as a chassis was demonstrated by substrate feeding and CDP-choline production. Overexpression of choline phosphate cytidylyltransferase and choline kinase enhanced the choline transformation pathway and improved the biosynthesis of CDP-choline. Furthermore, co-overexpression of ScHnm1, which is a heterologous choline transporter, highly improved the utilization of choline substrates, despite its easy degradation in cells. This strategy increased CDP-choline titer by 55-folds comparing with the wild-type (WT). Overexpression of cytidine-5′-monophosphate (CMP) kinase and CDP kinase in the CMP transformation pathway showed no positive effects. An increase in the ATP production by citrate stimulation or metabolic pathway modification further improved CDP-choline biosynthesis by 120%. Finally, the orthogonal optimization of key substrates and pH was carried out, and the resulting CDP-choline titer (6.0 g/L) at optimum conditions increased 88 times the original titer in the WT. This study provides a new paradigm for CDP-choline bioproduction by living cells.
SYN
Citicoline sodium is a chemically designate as Cytidine 5’-(trihydrogendiphosphate) P’-[2-(trimethylammonio) ethyl] ester monosodium salt, its molecular formula is C14H25N4NaO11P2 and molecular weight is 510.31(salt) and 488.32 (base- C14H26N4O11P2). It is a white crystalline, hygroscopic powder and readily soluble in water but practically insoluble in alcohol. Its melting point was 259 – 268°C and dissociation constant (Pka) was 4.4 [1]. Biopharmaceutical classification system (BCS) for Citicoline is Class – I (High solubility and High Permeability) [3]. Citicoline has a broad spectrum of therapeutic index, as a Neuroprotectant or Cerebroprotectant, in particular citicoline is useful the victims of ischemic stroke, head trauma and neurodegenerative disease. Citicoline is also used to treat unconsciousness resulting from cerebral thrombosis, hemorrhages, demyelinating diseases, cranial trauma and cerebropathies due to atherosclerosis [2]. Citicoline was originally developed in Japan for stroke. It was later introduced as a prescription drug in many European countries. In these countries it is now frequently prescribed for thinking problems related to circulation problems in the brain. In the US, citicoline is marketed as a dietary supplement [3]. Citicoline daily dosages may range from 250 mg to about 3000 mg and more preferably from 500 mg to about 2000 mg up to four or more times daily, duration of the treatment may vary from several weeks to several years, dosages may be varied over time depending on the severity of symptoms [4].

SYN
192/MUM/2012
The present invention discloses a novel, cost-effective process for preparing psychostimulant drug cytidinediphosphate-choline (CDP-Choline) commonly known as citicoline. The process comprises reacting cytidine 5-monophosphate with morpholine in presence of a coupling reagent and an organic solvent to form morpholidate compound; condensing morpholidate compound with calcium salt of phosphorylcholinehalide in presence of an acid to form citicoline calcium chloride; and purifying the citicoline calcium chlorideby passing through cationic and anionic resinsand eluting by water to form citicoline sodium of formula I.
Example:
(a) Preparation of citicoline calcium chloride:
S-Cytidine mono phosphate (1.25 kg)and morpholine (1.12 kg) were added into methanol {6.25 L) and DCC (1.50 kg) at 25 to 35 C.The reaction mixture was heated to 50 to 55 C and stirred for 7 hours. After completion of the reaction, the reaction mass was cooled to 25 to 35 C and the obtained reaction mass was added slowly to phosphoryl choline chloride calcium salt (1.9 kg) in methanol (8.75 L) solution. The pH was maintained to 3.8 to 4.2 using HC1 gas in IPA and stirred for 6 hours at 25 to 35 . The reaction mass was further heated to 45 to 5Q C. After completion of reaction the yeactkm mass was cooled and stirced for 1 hour. The product was filtered, washed with chilled methanol at 0 to 5 Cand suck dried to obtain citicoline calcium chloride.
Yield; 3.70-4.0 kg (b) Preparation of citicoline sodium:
The above obtained crude citicolinecalcium chloride was dissolved in water (6.25 L), filtered, washed with water and suck dried. Filtrate containing the product was re-filtered through Hyflo bed. The clear filtrate was eluted through column containing acidic cation exchange resins (12.5 L). The material was washed with water. The eluent was further passed through anion exchange resin (12.5 L). column and washed with water.
Complete aqueous solution after the passing through an-ion exchange resin was collected, pH of the solution was adjusted to 6.5 to 7.0 using 30 % sodium hydroxide solution (0.3Kg in 0.45L) and solution was concentrated using reverse osmosis. The solution was cooled to 25 to 35 C and charcoalated. The solution was filtered through hyflo bed at 25 to 35 C, washed with water. The solution was further filtered through ultra-filter at 25 to 35 C.
Clear filtrate and mixture of isopropanol and Methanol (1:1) (25 L) were stirred, the reaction mass was cooled to 0 to 5 C, and stirred for 2 hours. The product was filtered under nitrogen atmosphere, solid was washed with the mixture of IPA and methanol (1:1) (1.25 L) at 0 to 5 C and dried under vacuum below 95 C until moisture/LOD is less the 2.0%.
Yield: 1 to 1.2 kg

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
PATENT
https://patents.google.com/patent/CN1944661A/en
We Claim:
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Neurocoline |
| Other names | Cytidine diphosphate choline |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.346 |
| Chemical and physical data | |
| Formula | C14H27N4O11P2+ |
| Molar mass | 489.335 g·mol−1 |
| 3D model (JSmol) | |
| |
|
Use as a dietary supplement
Citicoline is available as a supplement in over 70 countries under a variety of brand names: Cebroton, Ceraxon, Cidilin, Citifar, Cognizin, Difosfocin, Hipercol, NeurAxon, Nicholin, Sinkron, Somazina, Synapsine, Startonyl, Trausan, Xerenoos, etc.[5] When taken as a supplement, citicoline is hydrolyzed into choline and cytidine in the intestine.[6] Once these cross the blood–brain barrier it is reformed into citicoline by the rate-limiting enzyme in phosphatidylcholine synthesis, CTP-phosphocholine cytidylyltransferase.[7][8]
Research
Memory and cognition
Studies have failed to confirm any potential benefits of citicoline for cognitive impairment.[9]
Ischemic stroke
Some preliminary research suggested that citicoline may reduce the rates of death and disability following an ischemic stroke.[10][11] However, the largest citicoline clinical trial to date (a randomised, placebo-controlled, sequential trial of 2298 patients with moderate-to-severe acute ischaemic stroke in Europe), found no benefit of administering citicoline on survival or recovery from stroke.[12] A meta-analysis of seven trials reported no statistically significant benefit for long-term survival or recovery.[13]
Vision
The effect of citicoline on visual function has been studied in patients with glaucoma, with possible positive effect for protecting vision.[14]
Mechanism of action
Neuroprotective effects
Citicoline may have neuroprotective effects due to its preservation of cardiolipin and sphingomyelin, preservation of arachidonic acid content of phosphatidylcholine and phosphatidylethanolamine, partial restoration of phosphatidylcholine levels, and stimulation of glutathione synthesis and glutathione reductase activity. Citicoline’s effects may also be explained by the reduction of phospholipase A2 activity.[15] Citicoline increases phosphatidylcholine synthesis.[16][17][18] The mechanism for this may be:
- By converting 1, 2-diacylglycerol into phosphatidylcholine
- Stimulating the synthesis of SAMe, which aids in membrane stabilization and reduces levels of arachidonic acid. This is especially important after an ischemia, when arachidonic acid levels are elevated.[19]
Neuronal membrane
The brain preferentially uses choline to synthesize acetylcholine. This limits the amount of choline available to synthesize phosphatidylcholine. When the availability of choline is low or the need for acetylcholine increases, phospholipids containing choline can be catabolized from neuronal membranes. These phospholipids include sphingomyelin and phosphatidylcholine.[15] Supplementation with citicoline can increase the amount of choline available for acetylcholine synthesis and aid in rebuilding membrane phospholipid stores after depletion.[20] Citicoline decreases phospholipase stimulation. This can lower levels of hydroxyl radicals produced after an ischemia and prevent cardiolipin from being catabolized by phospholipase A2.[21][22] It can also work to restore cardiolipin levels in the inner mitochondrial membrane.[21]
Cell signalling
Citicoline enhances cellular communication by increasing the availability of neurotransmitters, including acetylcholine, norepinephrine, and dopamine.[23] In simple terms, the choline component of citicoline is used to create acetylcholine, which is a primary executive neurotransmitter in the human brain. Clinical trials have found that citicoline supplementation improves attention, focus and learning in large part due to the increase in acetylcholine that results.[24]
Glutamate transport
Citicoline lowers increased glutamate concentrations and raises decreased ATP concentrations induced by ischemia. Citicoline also increases glutamate uptake by increasing expression of EAAT2, a glutamate transporter, in vitro in rat astrocytes. It is suggested that the neuroprotective effects of citicoline after a stroke are due in part to citicoline’s ability to decrease levels of glutamate in the brain.[25]
Pharmacokinetics
Citicoline is water-soluble, with more than 90% oral bioavailability.[20] Plasma levels peak one hour after oral ingestion, and a majority of the citicoline is excreted as CO2 in respiration, and again 24 hours after ingestion, where the remaining citicoline is excreted through urine.[26]
Side effects
Citicoline has a very low toxicity profile in animals and humans. Clinically, doses of 2000 mg per day have been observed and approved. Minor transient adverse effects are rare and most commonly include stomach pain and diarrhea.[17][unreliable medical source?] There have been suggestions that chronic citicoline use may have adverse psychiatric effects. However, a meta-analysis of the relevant literature does not support this hypothesis.[27][28] At most, citicoline may exacerbate psychotic episodes or interact with anti-psychotic medication.
Synthesis
In vivo
Phosphatidylcholine is a major phospholipid in eukaryotic cell membranes. Close regulation of its biosynthesis, degradation, and distribution is essential to proper cell function. Phosphatidylcholine is synthesized in vivo by two pathways
- The Kennedy pathway, which includes the transformation of choline to citicoline, by way of phosphorylcholine, to produce phosphatidylcholine when condensed with diacylglycerol.
- Phosphatidylcholine can also be produced by the methylation pathway, where phosphatidylethanolamine is sequentially methylated.[29]
References
- ^ Giménez R, Raïch J, Aguilar J (Nov 1991). “Changes in brain striatum dopamine and acetylcholine receptors induced by chronic CDP-choline treatment of aging mice”. British Journal of Pharmacology. 104 (3): 575–8. doi:10.1111/j.1476-5381.1991.tb12471.x. PMC 1908237. PMID 1839138.
- ^ Cavun S, Savci V (Oct 2004). “CDP-choline increases plasma ACTH and potentiates the stimulated release of GH, TSH and LH: the cholinergic involvement”. Fundamental & Clinical Pharmacology. 18 (5): 513–23. doi:10.1111/j.1472-8206.2004.00272.x. PMID 15482372. S2CID 33866107.
- ^ Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, Janssen OE, Schedlowski M, Elsenbruch S (Jun 2009). “Disturbed stress responses in women with polycystic ovary syndrome”. Psychoneuroendocrinology. 34 (5): 727–35. doi:10.1016/j.psyneuen.2008.12.001. PMID 19150179. S2CID 13202703.
- ^ Florio P, Zatelli MC, Reis FM, degli Uberti EC, Petraglia F (2007). “Corticotropin releasing hormone: a diagnostic marker for behavioral and reproductive disorders?”. Frontiers in Bioscience. 12: 551–60. doi:10.2741/2081. PMID 17127316.
- ^ Single-ingredient Preparations (: Citicoline). In: Martindale: The Complete Drug Reference [ed.by Sweetman S], 35th Ed. 2007, The Pharmaceutical Press: London (UK); e-version. .
- ^ Wurtman RJ, Regan M, Ulus I, Yu L (Oct 2000). “Effect of oral CDP-choline on plasma choline and uridine levels in humans”. Biochemical Pharmacology. 60 (7): 989–92. doi:10.1016/S0006-2952(00)00436-6. PMID 10974208.
- ^ Alvarez XA, Sampedro C, Lozano R, Cacabelos R (Oct 1999). “Citicoline protects hippocampal neurons against apoptosis induced by brain beta-amyloid deposits plus cerebral hypoperfusion in rats”. Methods and Findings in Experimental and Clinical Pharmacology. 21 (8): 535–40. doi:10.1358/mf.1999.21.8.794835. PMID 10599052.
- ^ Carlezon WA, Pliakas AM, Parow AM, Detke MJ, Cohen BM, Renshaw PF (Jun 2002). “Antidepressant-like effects of cytidine in the forced swim test in rats”. Biological Psychiatry. 51 (11): 882–9. doi:10.1016/s0006-3223(01)01344-0. PMID 12022961. S2CID 21170398.
- ^ Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC (2015). “The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives”. Clin Interv Aging. 10: 1421–9. doi:10.2147/CIA.S87886. PMC 4562749. PMID 26366063.
- ^ Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R (Nov 2000). “Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators”. Annals of Neurology. 48 (5): 713–22. doi:10.1002/1531-8249(200011)48:5<713::aid-ana4>3.0.co;2-#. PMID 11079534.
- ^ Saver JL (Fall 2008). “Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair”. Reviews in Neurological Diseases. 5 (4): 167–77. PMID 19122569.
- ^ Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, Serena J, Segura T, Cruz VT, Masjuan J, Cobo E, Secades JJ (Jul 2012). “Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial)”. Lancet. 380 (9839): 349–57. doi:10.1016/S0140-6736(12)60813-7. hdl:10400.10/663. PMID 22691567. S2CID 134947.
- ^ Shi PY, Zhou XC, Yin XX, Xu LL, Zhang XM, Bai HY (2016). “Early application of citicoline in the treatment of acute stroke: A meta-analysis of randomized controlled trials”. J. Huazhong Univ. Sci. Technol. Med. Sci. 36 (2): 270–7. doi:10.1007/s11596-016-1579-6. PMID 27072975. S2CID 25352343.
- ^ Roberti G, Tanga L, Michelessi M, Quaranta L, Parisi V, Manni G, Oddone F (2015). “Cytidine 5′-Diphosphocholine (Citicoline) in Glaucoma: Rationale of Its Use, Current Evidence and Future Perspectives”. Int J Mol Sci. 16 (12): 28401–17. doi:10.3390/ijms161226099. PMC 4691046. PMID 26633368.
- ^ Jump up to:a b Adibhatla RM, Hatcher JF, Dempsey RJ (Jan 2002). “Citicoline: neuroprotective mechanisms in cerebral ischemia”. Journal of Neurochemistry. 80 (1): 12–23. doi:10.1046/j.0022-3042.2001.00697.x. PMID 11796739.
- ^ López-Coviella I, Agut J, Savci V, Ortiz JA, Wurtman RJ (Aug 1995). “Evidence that 5′-cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels”. Journal of Neurochemistry. 65 (2): 889–94. doi:10.1046/j.1471-4159.1995.65020889.x. PMID 7616250. S2CID 10184322.
- ^ Jump up to:a b Conant R, Schauss AG (Mar 2004). “Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature”. Alternative Medicine Review. 9 (1): 17–31. PMID 15005642.
- ^ Babb SM, Wald LL, Cohen BM, Villafuerte RA, Gruber SA, Yurgelun-Todd DA, Renshaw PF (May 2002). “Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study”. Psychopharmacology. 161 (3): 248–54. doi:10.1007/s00213-002-1045-y. PMID 12021827. S2CID 28454793.
- ^ Rao AM, Hatcher JF, Dempsey RJ (Dec 1999). “CDP-choline: neuroprotection in transient forebrain ischemia of gerbils”. Journal of Neuroscience Research. 58 (5): 697–705. doi:10.1002/(sici)1097-4547(19991201)58:5<697::aid-jnr11>3.0.co;2-b. PMID 10561698.
- ^ Jump up to:a b D’Orlando KJ, Sandage BW (Aug 1995). “Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury”. Neurological Research. 17 (4): 281–4. doi:10.1080/01616412.1995.11740327. PMID 7477743.
- ^ Jump up to:a b Rao AM, Hatcher JF, Dempsey RJ (Mar 2001). “Does CDP-choline modulate phospholipase activities after transient forebrain ischemia?”. Brain Research. 893 (1–2): 268–72. doi:10.1016/S0006-8993(00)03280-7. PMID 11223016. S2CID 37271883.
- ^ Adibhatla RM, Hatcher JF (Aug 2003). “Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia”. Journal of Neuroscience Research. 73 (3): 308–15. doi:10.1002/jnr.10672. PMID 12868064. S2CID 17806057.
- ^ Secades JJ, Lorenzo JL (Sep 2006). “Citicoline: pharmacological and clinical review, 2006 update”. Methods and Findings in Experimental and Clinical Pharmacology. 28 Suppl B: 1–56. PMID 17171187.
- ^ Tardner, P. (2020-08-30). “The use of citicoline for the treatment of cognitive decline and cognitive impairment: A meta-analysis of pharmacological literature • International Journal of Environmental Science & Technology”. International Journal of Environmental Science & Technology. Retrieved 2020-08-31.
- ^ Hurtado O, Moro MA, Cárdenas A, Sánchez V, Fernández-Tomé P, Leza JC, Lorenzo P, Secades JJ, Lozano R, Dávalos A, Castillo J, Lizasoain I (Mar 2005). “Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport”. Neurobiology of Disease. 18 (2): 336–345. doi:10.1016/j.nbd.2004.10.006. PMID 15686962. S2CID 2818533.
- ^ Dinsdale JR, Griffiths GK, Rowlands C, Castelló J, Ortiz JA, Maddock J, Aylward M (1983). “Pharmacokinetics of 14C CDP-choline”. Arzneimittel-Forschung. 33 (7A): 1066–70. PMID 6412727.
- ^ Tardner, P. (2020-08-28). “Can Citicoline Cause Depression?: A review of the clinical literature • International Journal of Environmental Science & Technology”. International Journal of Environmental Science & Technology. Retrieved 2020-08-31.
- ^ Talih, Farid; Ajaltouni, Jean (2015). “Probable Nootropicinduced Psychiatric Adverse Effects: A Series of Four Cases”. Innovations in Clinical Neuroscience. 12 (11–12): 21–25. ISSN 2158-8333. PMC 4756795. PMID 27222762.
- ^ Fernández-Murray JP, McMaster CR (Nov 2005). “Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy pathway”. The Journal of Biological Chemistry. 280 (46): 38290–6. doi:10.1074/jbc.M507700200. PMID 16172116.
//////////CITOCOLINE, CDP-choline, Neuroprotective, ischemic stroke, head trauma,
C[N+](C)(C)CCOP(=O)(O)OP(=O)([O-])OCC1C(C(C(O1)N2C=CC(=NC2=O)N)O)O















![[(2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl [hydroxy-[2-(trimethylazaniumyl)ethoxy]phosphoryl] phosphate.png](https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=11583971&t=l)