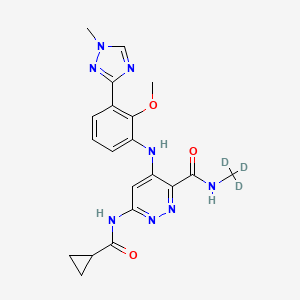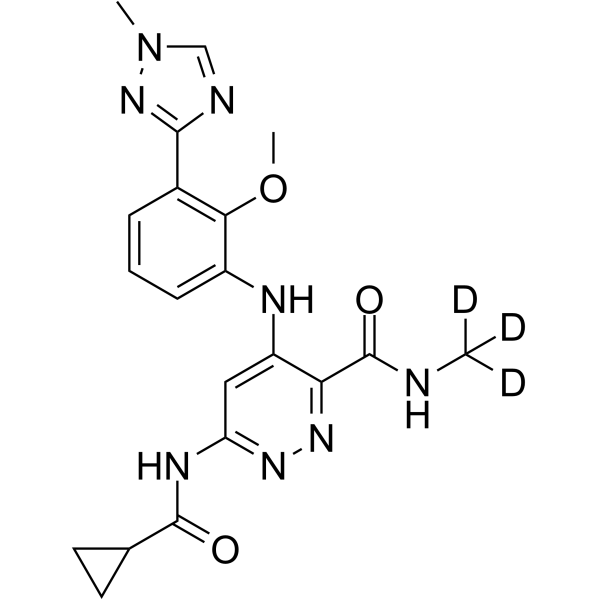
DEUCRAVACITINIB
BMS-986165
CAS 1609392-27-9, C20H22N8O3, 425.46
6-(cyclopropanecarbonylamino)-4-[2-methoxy-3-(1-methyl-1,2,4-triazol-3-yl)anilino]-N-(trideuteriomethyl)pyridazine-3-carboxamide
- OriginatorBristol-Myers Squibb
- ClassAmides; Aniline compounds; Anti-inflammatories; Antipsoriatics; Antirheumatics; Cyclopropanes; Ethers; Hepatoprotectants; Organic deuterium compounds; Pyridazines; Skin disorder therapies; Small molecules; Triazoles
- Mechanism of ActionTYK2 kinase inhibitors
- Phase IIIPlaque psoriasis
- Phase IICrohn’s disease; Lupus nephritis; Psoriatic arthritis; Systemic lupus erythematosus; Ulcerative colitis
- Phase IAutoimmune disorders
- No development reportedInflammatory bowel diseases; Psoriasis
- 02 Jul 2021Bristol-Myers Squibb plans a phase I pharmacokinetics trial (In volunteers) in USA (PO, Tablet) in July 2021 (NCT04949269)
- 14 Jun 2021Bristol-Myers Squibb plans a phase III trial for Psoriatic arthritis (Treatment-naïve) in USA, Brazil, Colombia, Czech republic, Hungary, Italy, Mexico, Romania, Spain and Taiwan in July 2021 (NCT04908202) (EudraCT2020-005097-10)
- 02 Jun 2021Interim efficacy and adverse events data from the phase III POETYK-PSO-1 trial in Psoriatic psoriasis presented at the 22nd Annual Congress of the European League Against Rheumatism (EULAR-2021)
BMS , presumed to be in collaboration with Jinan University and Chinese Academy of Sciences , is developing deucravacitinib, a TYK2 inhibitor, for treating autoimmune diseases, primarily psoriasis. In July 2021, deucravacitinib was reported to be in phase 3 clinical development.
Deucravacitinib (BMS-986165) is a highly selective, orally bioavailable allosteric TYK2 inhibitor for the treatment of autoimmune diseases, which selectively binds to TYK2 pseudokinase (JH2) domain (IC50=1.0 nM) and blocks receptor-mediated Tyk2 activation by stabilizing the regulatory JH2 domain. Deucravacitinib inhibits IL-12/23 and type I IFN pathways.
PAPER
https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.9b00444

Small molecule JAK inhibitors have emerged as a major therapeutic advancement in treating autoimmune diseases. The discovery of isoform selective JAK inhibitors that traditionally target the catalytically active site of this kinase family has been a formidable challenge. Our strategy to achieve high selectivity for TYK2 relies on targeting the TYK2 pseudokinase (JH2) domain. Herein we report the late stage optimization efforts including a structure-guided design and water displacement strategy that led to the discovery of BMS-986165 (11) as a high affinity JH2 ligand and potent allosteric inhibitor of TYK2. In addition to unprecedented JAK isoform and kinome selectivity, 11 shows excellent pharmacokinetic properties with minimal profiling liabilities and is efficacious in several murine models of autoimmune disease. On the basis of these findings, 11 appears differentiated from all other reported JAK inhibitors and has been advanced as the first pseudokinase-directed therapeutic in clinical development as an oral treatment for autoimmune diseases.
Bristol Myers Squibb Presents Positive Data from Two Pivotal Phase 3 Psoriasis Studies Demonstrating Superiority of Deucravacitinib Compared to Placebo and Otezla® (apremilast)
Significantly more patients treated with deucravacitinib achieved PASI 75 and sPGA 0/1 compared to patients treated with placebo and Otezla at Week 16, with an increased benefit versus Otezla at Week 24 and maintained through Week 52
Deucravacitinib was well tolerated with a low rate of discontinuation due to adverse events
Deucravacitinib is a first-in-class, oral, selective tyrosine kinase 2 (TYK2) inhibitor with a unique mechanism of action
Results presented as late-breaking research at the 2021 American Academy of Dermatology Virtual Meeting Experience
PRINCETON, N.J.–(BUSINESS WIRE)– Bristol Myers Squibb (NYSE:BMY) today announced positive results from two pivotal Phase 3 trials evaluating deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, for the treatment of patients with moderate to severe plaque psoriasis. The POETYK PSO-1 and POETYK PSO-2 trials, which evaluated deucravacitinib 6 mg once daily, met both co-primary endpoints versus placebo, with significantly more patients achieving Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment score of clear or almost clear (sPGA 0/1) after 16 weeks of treatment with deucravacitinib. Deucravacitinib was well tolerated with a low rate of discontinuation due to adverse events (AEs).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210423005134/en(Graphic: Business Wire)
Deucravacitinib demonstrated superior skin clearance compared with Otezla® (apremilast) for key secondary endpoints in both studies, as measured by PASI 75 and sPGA 0/1 responses at Week 16 and Week 24. Findings include:
PASI 75 Response in POETYK PSO-1 and POETYK PSO-2:
- At Week 16, 58.7% and 53.6% of patients receiving deucravacitinib achieved PASI 75 response, respectively, versus 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving Otezla.
- At Week 24, 69.0% and 59.3% of patients receiving deucravacitinib achieved PASI 75 response, respectively, versus 38.1% and 37.8% receiving Otezla.
- Among patients who achieved PASI 75 response at Week 24 with deucravacitinib and continued treatment with deucravacitinib, 82.5% and 81.4%, respectively, maintained PASI 75 response at Week 52.
sPGA 0/1 Response in POETYK PSO-1 and POETYK PSO-2:
- At Week 16, 53.6% and 50.3% of patients receiving deucravacitinib achieved sPGA 0/1 response, respectively, versus 7.2% and 8.6% receiving placebo and 32.1% and 34.3% receiving Otezla.
- At Week 24, 58.4% and 50.4% of patients receiving deucravacitinib achieved sPGA 0/1 response, respectively, versus 31.0% and 29.5% receiving Otezla.
“In both pivotal studies, deucravacitinib was superior to Otezla across multiple endpoints, including measures of durability and maintenance of response, suggesting that deucravacitinib has the potential to become a new oral standard of care for patients who require systemic therapy and need a better oral option for their moderate to severe plaque psoriasis,” said April Armstrong, M.D., M.P.H., Associate Dean and Professor of Dermatology at the University of Southern California. “As many patients with moderate to severe plaque psoriasis remain undertreated or even untreated, it is also highly encouraging to see that deucravacitinib improved patient symptoms and outcomes to a greater extent than Otezla.”
Superiority of Deucravacitinib Versus Placebo and Otezla
Deucravacitinib demonstrated a robust efficacy profile, including superiority to placebo for the co-primary endpoints and to Otezla for key secondary endpoints. In addition to PASI 75 and sPGA 0/1 measures, deucravacitinib was superior to Otezla across both studies in multiple other secondary endpoints, demonstrating significant and clinically meaningful efficacy improvements in symptom burden and quality of life measures.
|
POETYK PSO-1 and POETYK PSO-2 Results at Week 16 and Week 24 |
||||||
|
Endpoint |
POETYK PSO-1 (n=666) |
POETYK PSO-2 (n=1,020) |
||||
|
Deucravacitinib 6 mg (n=332) |
Otezla 30 mg (n=168) |
Placebo (n=166) |
Deucravacitinib 6 mg (n=511) |
Otezla 30 mg (n=254) |
Placebo (n=255) |
|
|
PASI 75*a |
||||||
|
Week 16 |
58.7%*† |
35.1% |
12.7% |
53.6%*‡ |
40.2% |
9.4% |
|
Week 24 |
69.0%† |
38.1% |
– |
59.3%† |
37.8% |
– |
|
sPGA 0/1*b |
||||||
|
Week 16 |
53.6%*† |
32.1% |
7.2% |
50.3%*† |
34.3% |
8.6% |
|
Week 24 |
58.4%† |
31.0% |
– |
50.4%† |
29.5% |
– |
|
(Scalp) ss-PGA 0/1c |
||||||
|
Week 16 |
70.8%*† |
39.1% |
17.4% |
60.3%*† |
37.3% |
17.3% |
|
Week 24 |
71.8%† |
42.7% |
– |
59.7%‡ |
41.6% |
– |
|
PSSD-Symptoms CFBd |
||||||
|
Week 16 |
-26.7*† |
-17.8 |
-3.6 |
-28.3*† |
-21.1 |
-4.7 |
|
Week 24 |
-31.9† |
-20.7 |
– |
-29.1† |
-21.4 |
– |
|
DLQI 0/1e |
||||||
|
Week 16 |
40.7%*† |
28.6% |
10.6% |
38.0%*† |
23.1% |
9.8% |
|
Week 24 |
47.8%‡ |
24.2% |
– |
41.8%† |
21.5% |
– |
|
*Co-primary endpoints for POETYK PSO-1 and POETYK PSO-2 were PASI 75 and sPGA 0/1 for deucravacitinib vs placebo at Week 16. |
|
a. PASI 75 is defined as at least a 75% improvement from baseline in Psoriasis Area and Severity Index (PASI) scores. *p<0.0001 vs placebo. †p<0.0001 vs Otezla. ‡p=0.0003 vs Otezla. |
|
b. sPGA 0/1 is defined as a static Physician’s Global Assessment (sPGA) score of clear or almost clear. *p<0.0001 vs placebo. †p<0.0001 vs Otezla. |
|
c. ss-PGA 0/1 is defined as a scalp-specific Physician’s Global Assessment (ss-PGA) score of clear or almost clear in those with ss-PGA of at least 3 (moderate) at baseline. POETYK PSO-1: *p<0.0001 vs placebo. †p<0.0001 vs Otezla. POETYK PSO-2: *p<0.0001 vs placebo. †p<0.0001 vs Otezla. ‡p=0.0002 vs Otezla. |
|
d. Change from baseline (CFB) in Psoriasis Symptoms and Signs Diary (PSSD) captures improvement in symptoms of itch, pain, stinging, burning and skin tightness in patient eDiaries. *p<0.0001 vs placebo. †p<0.0001 vs Otezla. |
|
e. Dermatology Life Quality Index (DLQI) 0/1 scores reflect no effect at all on patient’s life in patients with a baseline DLQI score of ≥2. POETYK PSO-1: *p<0.0001 vs placebo. †p=0.0106 vs Otezla. ‡p<0.0001 vs Otezla. POETYK PSO-2: *p<0.0001 vs placebo. †p<0.0001 vs Otezla. |
Safety and Tolerability
Deucravacitinib was well-tolerated and had a similar safety profile in both trials. At Week 16, 2.9% of 419 patients on placebo, 1.8% of 842 patients on deucravacitinib and 1.2% of 422 patients on Otezla experienced serious adverse events (SAEs) across both studies. The most common AEs (≥5%) with deucravacitinib treatment at Week 16 were nasopharyngitis and upper respiratory tract infection with low rates of headache, diarrhea and nausea. At Week 16, 3.8% of patients on placebo, 2.4% of patients on deucravacitinib and 5.2% of patients on Otezla experienced AEs leading to discontinuation. Across POETYK PSO-1 and POETYK PSO-2 over 52 weeks, SAEs when adjusted for exposure (exposure adjusted incidence per 100 patient-years [EAIR]) were 5.7 with placebo, 5.7 with deucravacitinib and 4.0 with Otezla. In the same timeframe across both studies, EAIRs for AEs leading to discontinuation were 9.4 with placebo, 4.4 with deucravacitinib and 11.6 with Otezla. No new safety signals were observed during Weeks 16‒52.
Across both Phase 3 trials, rates of malignancy, major adverse cardiovascular events (MACE), venous thromboembolism (VTE) and serious infections were low and generally consistent across active treatment groups. No clinically meaningful changes were observed in multiple laboratory parameters (including anemia, blood cells, lipids and liver enzymes) over 52 weeks.
“The findings from both studies affirm that deucravacitinib – a first-in-class, oral, selective TYK2 inhibitor with a unique mechanism of action that inhibits the IL-12, IL-23 and Type 1 IFN pathways –may become an oral treatment of choice for people living with psoriasis. We believe deucravacitinib has significant potential across a broad range of immune-mediated diseases, and we are committed to further advancing our expansive clinical program with this agent,” said Mary Beth Harler, M.D., head of Immunology and Fibrosis Development, Bristol Myers Squibb. “We are in discussions with health authorities with the goal of bringing this new therapy to appropriate patients as soon as possible. At Bristol Myers Squibb, we are committed to building an immunology portfolio that addresses pressing unmet needs that exist for those impacted by serious dermatologic conditions and other immune-mediated diseases, to ultimately deliver the promise of living a better life.”
These results are available as a late-breaking research presentation (Session S033 – Late-Breaking Research Abstracts) as part of the 2021 American Academy of Dermatology (AAD) Virtual Meeting Experience (VMX). Full results of both studies will be submitted to a medical journal for peer review. In November 2020 and February 2021, respectively, Bristol Myers Squibb announced positive topline results from POETYK PSO-1 and POETYK PSO-2.
Visit www.bms.com/media/medical-meetings/bms-at-aad-vmx.html for more information on Bristol Myers Squibb’s scientific approach and resources on psoriasis and immune-mediated diseases.
About Deucravacitinib
Deucravacitinib (pronounced doo-krav-a-sih-ti-nib) is a first-in-class, oral, selective tyrosine kinase 2 (TYK2) inhibitor with a unique mechanism of action. Deucravacitinib is the first and only TYK2 inhibitor in clinical studies across multiple immune-mediated diseases. Bristol Myers Squibb scientists designed deucravacitinib to selectively target TYK2, thereby inhibiting signaling of interleukin (IL)-12, IL-23 and Type 1 interferon (IFN), key cytokines involved in psoriasis pathogenesis. Deucravacitinib achieves a high degree of selectivity by uniquely binding to the regulatory, rather than the active, domain of TYK2, which is structurally distinct from the regulatory domains of Janus kinase (JAK) 1, 2 and 3. At therapeutic doses, deucravacitinib does not inhibit JAK1, JAK2 or JAK3. Due to the innovative design of deucravacitinib, Bristol Myers Squibb earned recognition with the 2019 Thomas Alva Edison Patent Award for the science underpinning the clinical development of deucravacitinib.
Deucravacitinib is being studied in multiple immune-mediated diseases, including psoriasis, psoriatic arthritis, lupus and inflammatory bowel disease. In addition to POETYK PSO-1 and POETYK PSO-2, Bristol Myers Squibb is evaluating deucravacitinib in three other Phase 3 studies in psoriasis: POETYK PSO-3 (NCT04167462); POETYK PSO-4 (NCT03924427); POETYK PSO-LTE (NCT04036435). Deucravacitinib is not approved for any use in any country.
About the Phase 3 POETYK PSO-1 and POETYK PSO-2 Studies
PrOgram to Evaluate the efficacy and safety of deucravacitinib, a selective TYK2 inhibitor (POETYK) PSO-1 (NCT03624127) and POETYK PSO-2 (NCT03611751) are global Phase 3 studies designed to evaluate the safety and efficacy of deucravacitinib compared to placebo and Otezla® (apremilast) in patients with moderate to severe plaque psoriasis. Both POETYK PSO-1, which enrolled 666 patients, and POETYK PSO-2, which enrolled 1,020 patients, were multi-center, randomized, double-blind trials that evaluated deucravacitinib (6 mg once daily) compared with placebo and Otezla (30 mg twice daily). POETYK PSO-2 included a randomized withdrawal and retreatment period after Week 24.
The co-primary endpoints of both POETYK PSO-1 and POETYK PSO-2 were the percentage of patients who achieved Psoriasis Area and Severity Index (PASI) 75 response and those who achieved static Physician’s Global Assessment (sPGA) score of 0 or 1 at Week 16 versus placebo. Key secondary endpoints of the trials included the percentage of patients who achieved PASI 75 and sPGA 0/1 compared to Otezla at Week 16 and other measures.
About Psoriasis
Psoriasis is a widely prevalent, chronic, systemic immune-mediated disease that substantially impairs patients’ physical health, quality of life and work productivity. Psoriasis is a serious global problem, with at least 100 million people worldwide impacted by some form of the disease, including around 14 million people in Europe and approximately 7.5 million people in the United States. Up to 90 percent of patients with psoriasis have psoriasis vulgaris, or plaque psoriasis, which is characterized by distinct round or oval plaques typically covered by silvery-white scales. Despite the availability of effective systemic therapy, many patients with moderate to severe psoriasis remain undertreated or even untreated and are dissatisfied with current treatments. People with psoriasis report an impact on their emotional well-being, straining both personal and professional relationships and causing a reduced quality of life. Psoriasis is associated with multiple comorbidities that may impact patients’ well-being, including psoriatic arthritis, cardiovascular disease, metabolic syndrome, obesity, diabetes, inflammatory bowel disease and depression.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol Myers Squibb, visit us at BMS.com or follow us on LinkedIn, Twitter, YouTube, Facebook and Instagram.
Celgene and Juno Therapeutics are wholly owned subsidiaries of Bristol-Myers Squibb Company. In certain countries outside the U.S., due to local laws, Celgene and Juno Therapeutics are referred to as, Celgene, a Bristol Myers Squibb company and Juno Therapeutics, a Bristol Myers Squibb company.
Otezla® (apremilast) is a registered trademark of Amgen Inc.
PATENT
WO-2021129467
Novel crystalline polymorphic forms (CSI and CSII) of deucravacitinib (also known as BMS-986165), useful a tyrosine kinase 2 pseudokinase domain (TYK2) inhibitor for treating psoriasis, systemic lupus erythematosus, and Crohn’s disease.
PATENT
US9505748 , a family member of WO2014074661 .
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014074661
Preparation 1
Step l Int1
Step 2 Int2 Step 3 Int3 Step 4 Int4
Example 52
Step 1
[00219] To a solution of 2-methoxy-3-(l-methyl-lH-l ,2,4-triazol-3-yl)aniline (10.26 g, 50.2 mmol) and Int8 (10.5 g, 50.2 mmol) in THF (120 mL) was added lithium bis(trimethylsilyl)amide (LiHMDS, 1M in THF, 151 mL, 151 mmol) in a dropwise manner using a pressure equalized addition funnel. The reaction was run for 10 minutes after the completion of the addition and then quenched with HCl (1M aq., 126 mL, 126 mmol). The reaction was concentrated on a rotary evaporator until the majority of the THF was removed and a precipitate prevailed throughout the vessel. Water (-500 mL) was then added and the slurry sonicated for 5 minutes and stirred for 15 min. The solid was filtered off, rinsing with water and then air dried for 30 minutes. The powder was collected and dissolved in dichloromethane. The organic layer was washed with water and brine and then dried over sodium sulfate, filtered and concentrated to provide the product (12.5 g, 66% yield) (carried on as is). 1H NMR (400MHz, DMSO-d6) δ 11.11 (s, 1H), 9.36 (s, 1H), 8.56 (s, 1H), 7.72 (dd, J=7.8, 1.6 Hz, 1H), 7.60 (dd, J=7.9, 1.5 Hz, 1H), 7.29 (t, J=7.9 Hz, 1H), 7.19 (s, 1H), 3.95 (s, 3H), 3.72 (s, 3H). LC retention time 1.18 [E]. MS(E+) m/z: 377 (MH+).
Step 2
[00220] Intl3 (2.32 g, 6.16 mmol) and cyclopropanecarboxamide (1.048 g, 12.31 mmol) were dissolved in dioxane (62 mL) and Pd2(dba)3 (564 mg, 0.616 mmol), Xantphos (534 mg, 0.924 mmol) and cesium carbonate (4.01 g, 12.3 mmol) were added. The vessel was evacuated three times (backfilling with nitrogen) and then sealed and heated to 130 °C for 140 minutes. The reaction was filtered through CELITE® (eluting with ethyl acetate) and concentrated (on smaller scale this material could then be purified using preparative HPLC). The crude product was adsorbed onto CELITE® using dichloromethane, dried and purified using automated chromatography (100% EtOAc) to provide example 52 (1.22 g, 46% yield). 1H NMR (500MHz, chloroform-d) δ 10.99 (s, 1H), 8.63 (s, 1H), 8.18 (s, 1H), 8.10 (d, J=0.5 Hz, 2H), 7.81 (dd, J=7.9, 1.7 Hz, 1H), 7.51 (dd, J=7.9, 1.4 Hz, 1H), 7.33 – 7.20 (m, 7H), 4.01 (d, J=0.3 Hz, 3H), 3.82 (s, 3H), 1.73 -1.60 (m, 1H), 1.16 – 1.06 (m, 2H), 0.97 – 0.84 (m, 2H). LC retention time 6.84 [N]. MS(E+) m/z: 426 (MH+).
Example 53
[00221] To a homogeneous solution of Example 52 (50 mg, 0.12 mmol) in dichloromethane (3 mL) was added HCI (1M aq., 0.13 mL, 0.13 mmol) resulting in the solution turning yellow. The homogenous solution was concentrated down and then re-concentrated from dichloromethane twice to remove residual water, resulting in a white powder. The powder was suspended in dichloromethane and sonicated for 15 minutes, the powder was then collected via filtration, rinsing with dichloromethane to provide the corresponding HCI salt (38 mg, 70% yield). 1H NMR (500MHz, chloroform-d) δ 12.02 (s, 1H), 8.35 (s, 1H), 8.16 (s, 1H), 8.01 (dd, J=7.9, 1.5 Hz, 1H), 7.57 (br. s., 1H), 7.52 -7.46 (m, 1H), 7.36 (t, J=7.9 Hz, 1H), 4.03 (s, 3H), 3.83 (s, 3H), 2.05 – 1.95 (m, 1H), 1.16 – 1.09 (m, 2H), 1.03 (dd, J=7.4, 3.6 Hz, 2H). LC retention time 0.62 [j]. MS(E+) m/z: 426 (MH+).
[00222] Compare to NMR of parent free base: 1H NMR (500MHz, chloroform-d) δ 10.99 (s, 1H), 8.63 (s, 1H), 8.18 (s, 1H), 8.10 (d, J=0.5 Hz, 2H), 7.81 (dd, J=7.9, 1.7 Hz, 1H), 7.51 (dd, J=7.9, 1.4 Hz, 1H), 7.33 – 7.20 (m, 7H), 4.01 (d, J=0.3 Hz, 3H), 3.82 (s, 3H), 1.73 – 1.60 (m, 1H), 1.16 – 1.06 (m, 2H), 0.97 – 0.84 (m, 2H).
////////////DEUCRAVACITINIB, phase 3, BMS-986165, BMS 986165, psoriasis, systemic lupus erythematosus, Crohn’s disease,
CNC(=O)C1=NN=C(C=C1NC2=CC=CC(=C2OC)C3=NN(C=N3)C)NC(=O)C4CC4