
IIBR-100
Brilife
Recombinant vesicular stomatitis virus (rVSV) vaccine
Israel Institute for Biological Research
Hadassah Medical Center; Sheba Medical Center Hospital
The SARS-CoV-2 virus is responsible for the COVID-19 pandemic. The pandemic emerged from Wuhan Province in China in December 2019 and was declared by the WHO Director-General a Public Health Emergency of International Concern on 30 January 2020.
In this study, a vaccine developed by IIBR for SARS-CoV-2 virus will be assessed for its safety and potential efficacy in volunteers. The study is comprised of two phases, a dose-escalation phase (phase I) during which subjects (18-55 years old) will be randomly allocated to receive a single administration of IIBR-100 100 at low, mid or high dose or saline or two administrations of IIBR-100 at low dose, or saline, 28 days apart.
Based on results obtained during phase I, and cumulative phase I data review, the expansion phase (phase II) has begun, during which larger cohorts as well as elderly age subjects will be randomly allocated to receive a single administration of IIBR-100 at low, mid or high dose or saline, or two administrations of IIBR-100 at low, mid or high dose (prime-boost) or saline, 28 days apart. Additional top-dose (prime-boost) may be implemented when immunogenicity of any prime-boost arm is considered insufficient.
Based on immunogenicity preliminary data and DSMB recommendations, the two administrations of mid, high and top dose (prime-boost) or saline will continue.
The subjects will be followed for a period of up to 12 months post last vaccine administration to assess the safety and efficacy of the vaccine.
https://clinicaltrials.gov/ct2/show/NCT04608305
IIBR-100 also known as Brilife is a COVID-19 vaccine candidate developed by The Israel Institute for Biological Research.[1][2]
References
- ^ Clinical trial number NCT04608305 for “Phase I/II Randomized, Multi-Center, Placebo-Controlled, Dose-Escalation Study to Evaluate the Safety, Immunogenicity and Potential Efficacy of an rVSV-SARS-CoV-2-S Vaccine (IIBR-100) in Adults” at ClinicalTrials.gov
- ^ Jeffay N (29 December 2020). “As Israel goes vaccine-wild, will the homegrown version lose its shot?”. The Times of Israel. Retrieved 1 January 2021.
candidate developed by The Israel Institute for Biological Research.[1][2]
Israeli institute’s COVID vaccine candidate said very effective in animal trials
Secretive Israeli research center’s shot shows near 100% efficacy in non-human trials, is on par with US company Moderna’s candidate, TV report says
Israeli researchers at a top secret research center have made progress on a coronavirus vaccine that shows a high level of effectiveness in animals, according to a Friday TV report.
However, there is no guarantee that the vaccine under development will be effective in humans, or will be available soon.
The Israel Institute for Biological Research (IIBR), a secretive unit that works under the Prime Minister’s Office, developed a vaccine that shows close to 100 percent protection against the virus in lab animals, the Channel 12 report said, citing “a security source.”
The vaccine under development is on par in effectiveness with a vaccine being developed by US biotechnology company Moderna, the report said.
Unlike vaccines developed abroad, the domestic vaccine will first be delivered to Israeli citizens, it added. If successful, it was expected to provide protection against the disease with a single dose.
The institute has not started human trials but was preparing to manufacture 10 to 15 million doses, report said.
Hebrew media have reported on potential breakthroughs at the shadowy institute several times before, starting in mid-March, with the Defense Ministry pushing back on some of the claims to tamper expectations.

IIBR said last month that it had completed successful coronavirus vaccine trials on rodents, paving the way for further testing on other animals and then possibly human trials.
In a paper published on the website of bioRxiv, an online repository for papers that haven’t yet been peer-reviewed, the institute, which is based in Ness Ziona, said it hopes to have a finished vaccine in a year, or possibly even earlier.
In the abstract of the report, the researchers say their vaccine, which they tested on hamsters, “results in rapid and potent induction of neutralizing antibodies against SARS-CoV-2,” the virus that causes COVID-19.
Earlier this month a vaccine adviser to the government cautioned that there was no guarantee that the shots being developed will prove widely effective.
In May, the institute confirmed that it had isolated an antibody it believed could be used to develop treatments against the virus. The development would not be useful in the creation of a vaccine, but would rather be a move toward a drug treatment for those who have already contracted the disease.
Tal Zaks, Moderna’s Israeli chief medical officer, described to Channel 12 on Friday the company’s push into Phase 3 testing of its vaccine candidate, which was developed with the National Institutes of Health, and began its first injections Monday.
The trial, the world’s largest vaccine study, plans to test the vaccine on 30,000 volunteers.
There’s still no guarantee that the experimental vaccine, developed by the National Institutes of Health and Moderna Inc., will really offer protection.
“The first time we saw the first model, that the vaccine, even if it’s just in mice, successfully stimulated the immune system to identify the virus and neutralize it, I knew that we hadn’t missed anything, that we had the correct vaccine,” he said.
“And of course the second ‘ah-ha’ moment was when we saw the first clinical results, when it was clear that in humans we weren’t just getting to antibody levels we were seeing in sick people, which is what we aspired to, but we were getting to even higher levels,” Zaks said.
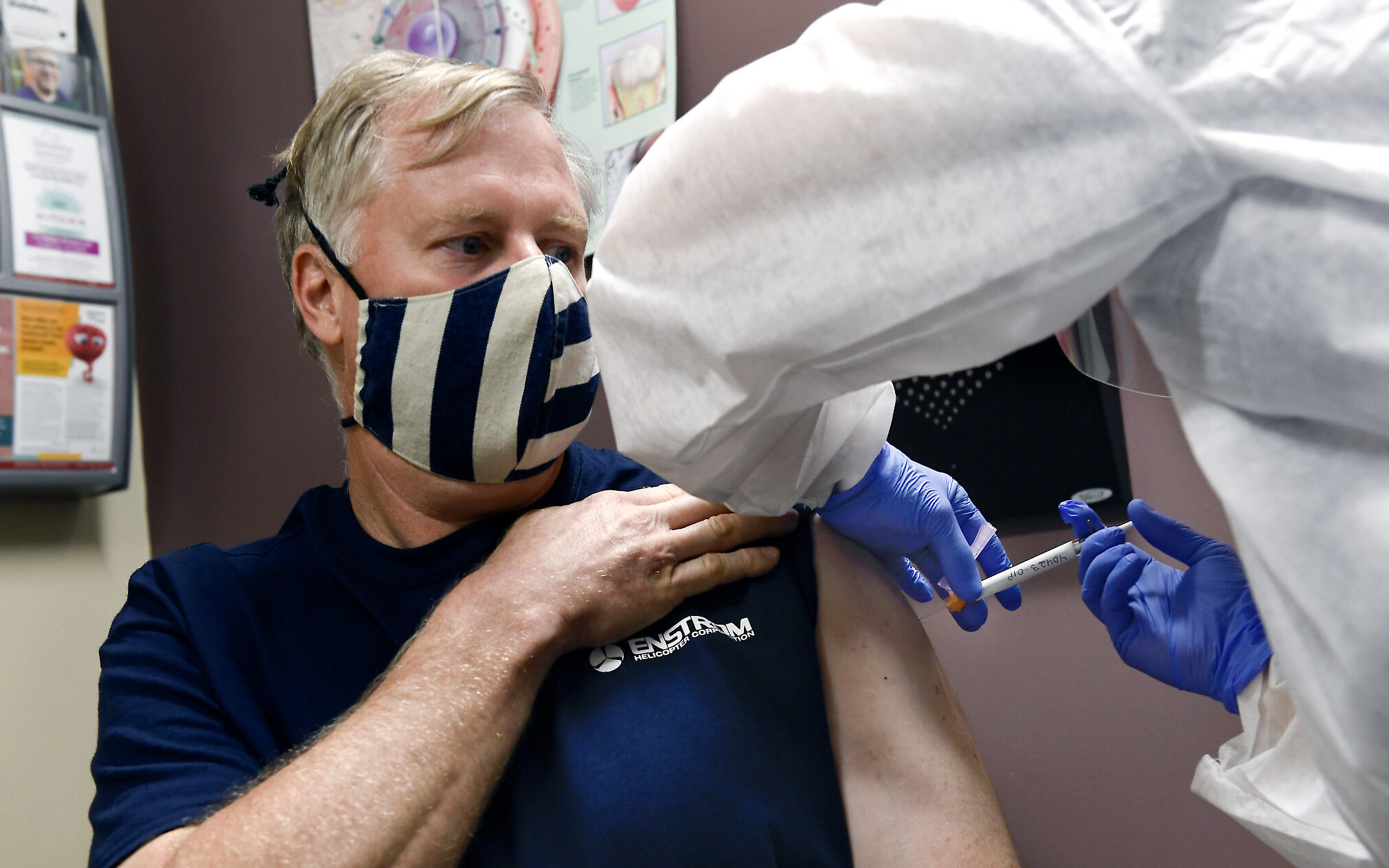
Last month Israel signed a deal with Moderna for the potential purchase of its coronavirus vaccine if it ends up proving effective.
Moderna said the vaccination was administered in Savannah, Georgia, the first site to get underway among more than seven dozen trial sites scattered around the country.
Several other vaccines made by China and by Britain’s Oxford University earlier this month began smaller final-stage tests in Brazil and other hard-hit countries.
The massive studies aren’t just to test if the shots work — they’re needed to check each potential vaccine’s safety. And following the same study rules will let scientists eventually compare all the shots.
It normally takes years to create a new vaccine from scratch, but scientists are setting speed records this time around, spurred by knowledge that vaccination is the world’s best hope against the pandemic.
If everything goes right with the final studies, it still will take months for the first data to trickle in from the Moderna test, followed by the Oxford one.
Governments around the world are trying to stockpile millions of doses of those leading candidates so if and when regulators approve one or more vaccines, immunizations can begin immediately. But the first available doses will be rationed, presumably reserved for people at highest risk from the virus.
Coronavirus cases in Israel rose by 1,791 in 24 hours on Friday and the national death toll hit 512, according to the latest Health Ministry figures.
The total case count stood at 70,970, with 320 patients in serious condition, including 98 on ventilators. The number of recovered patients reached 43,850.
Israel has the fifth-highest number of new coronavirus infections per capita in the world, overtaking the United States, according to data compiled by a scientific publication based at Oxford University.
And while Israel has seen the number of new coronavirus cases rocket to more than 2,000 a day in recent weeks, a new Hebrew University report published on Thursday asserted that Israel has managed to gain control of the second wave of the coronavirus, thanks to a recent stabilization in the number of seriously and moderately ill patients.
The curve for seriously and moderately ill patients began to spike in late June before stabilizing in recent days, the researchers reported. They credited the restrictions imposed by the government in recent weeks to limit crowding for helping to flatten the curve.
According to the report, the death toll will climb by roughly 200 in the coming three weeks as a result of the high infection rate over the past month.
Experts have blamed a too-speedy reopening and the lack of an effective contact-tracing program as main factors in the virus resurgence, which has come as new daily coronavirus cases around the world have also reached record highs.
| Vaccine description | |
|---|---|
| Target | SARS-CoV-2 |
| Vaccine type | Viral vector |
| Clinical data | |
| Other names | Brilife |
| Routes of administration |
Intramuscular |
| Part of a series on the |
| COVID-19 pandemic |
|---|
|
//////IIBR-100, Brilife, COVID-19, vaccine, israel, corona virus, covid 19, SARS-CoV-2















